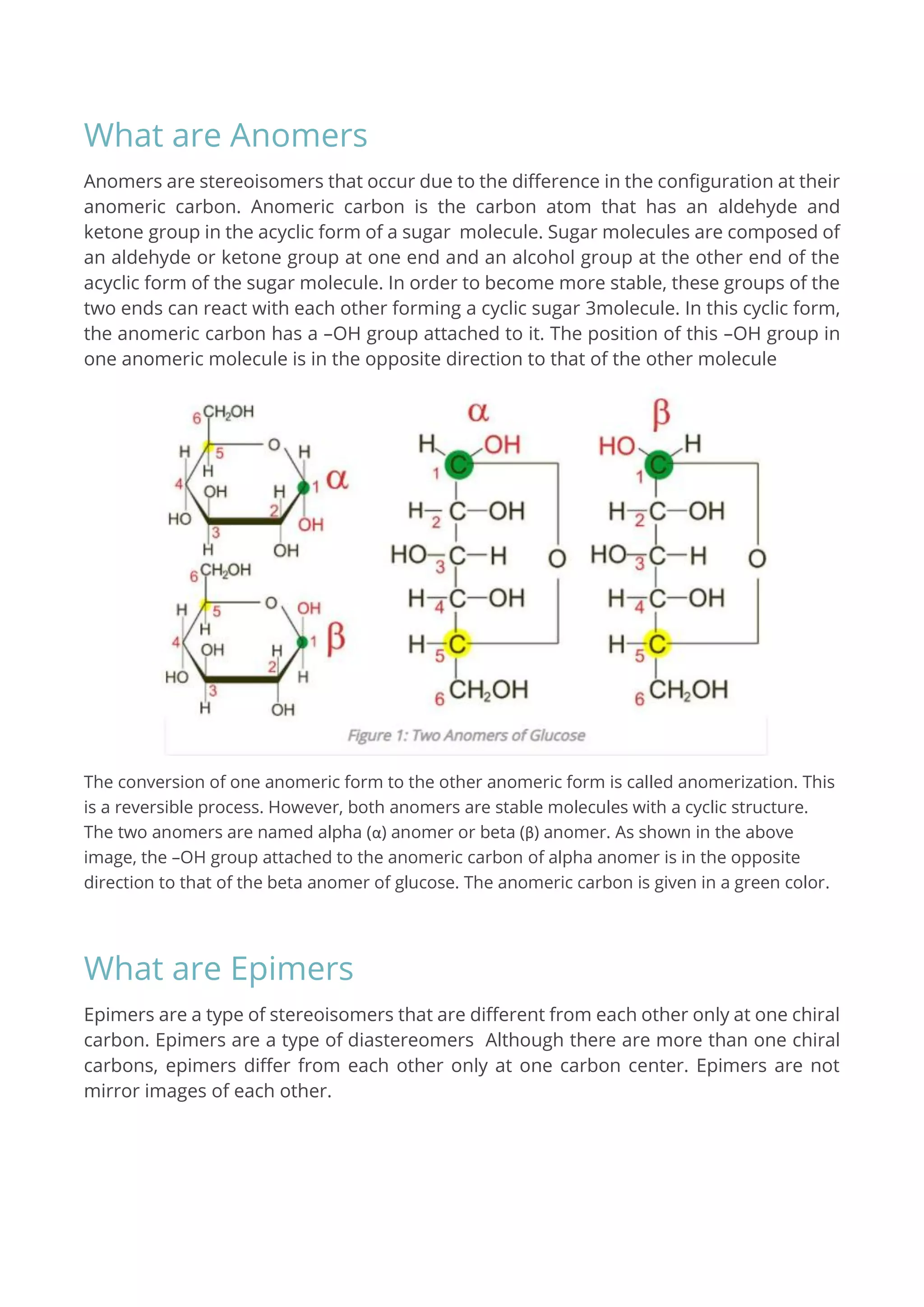
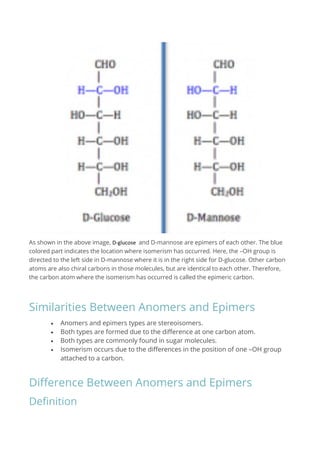
Anomers are stereoisomers differing in configuration at the anomeric carbon, typically found in cyclic sugar molecules, while epimers differ at only one chiral carbon. Both types are formed due to variations in the position of an –OH group and are commonly associated with sugar structures. The key distinction is that anomers specifically involve the anomeric carbon, whereas epimers can be either acyclic or cyclic.