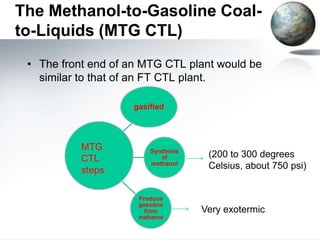
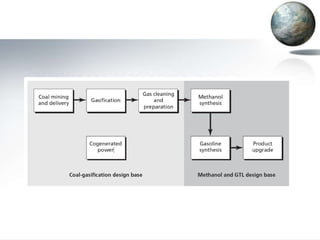
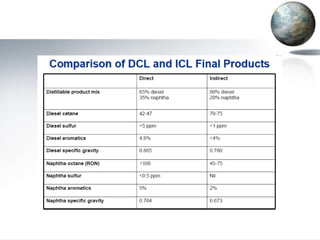
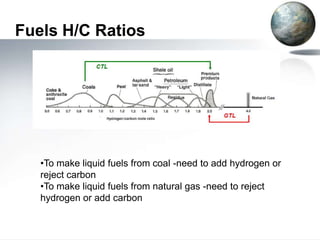
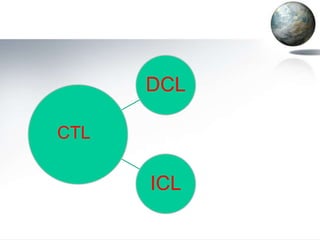
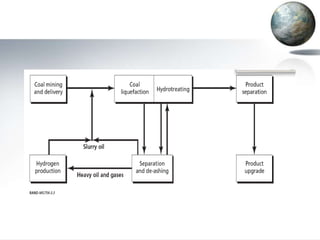
This document discusses different methods of coal liquefaction, which is the process of converting coal into liquid fuels. There are three main types of coal liquefaction: direct coal liquefaction, indirect coal liquefaction, and methanol-to-gasoline coal-to-liquids. Direct coal liquefaction involves adding hydrogen to coal under heat and pressure to increase its hydrogen-to-carbon ratio. Indirect coal liquefaction involves gasifying coal to produce syngas, then using Fischer-Tropsch chemistry to convert the syngas to liquid fuels. Methanol-to-gasoline coal-to-liquids first produces methanol from syngas, then converts the methanol into gasoline. Each method aims to produce liquid fuels







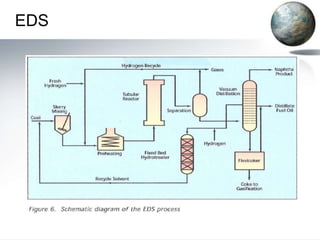






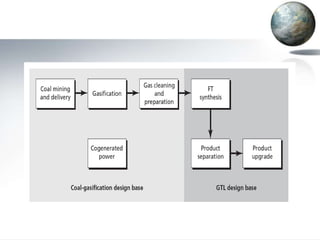
![• The process begins with the gasification of coal
• reacting coal with steam and oxygen at elevated
temperatures (1,000 to 1,500 degrees Celsius)
and moderate pressure(~500 pounds per square
inch [psi])
• The naphtha product is basically a very low–
octane (i.e., about 40 octane) gasoline that must
be extensively upgraded before it can be used as
an automotive fuel.
• Conversion of synthesis gas to hydrocarbons is
highly exothermic (i.e., it releases heat).](https://image.slidesharecdn.com/coalliquefaction-120508130128-phpapp01/85/Coal-liquefaction-22-320.jpg)