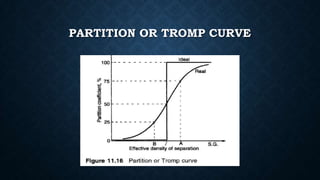
This document provides an overview of dense medium separation. It begins by defining dense medium separation as a process that uses heavy liquids to separate minerals of different specific gravities, with those lighter than the liquid floating and those denser sinking. It then discusses the principle behind dense medium separation and different types of dense mediums that can be used, including suspensions of solids in water. The document also covers characteristics of dense medium separation such as its applications in coal preparation and mineral processing as well as factors that influence its separation efficiency.