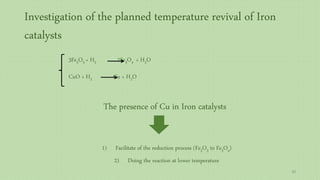
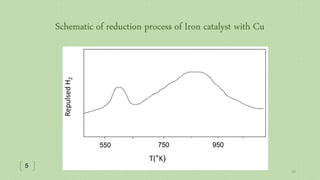
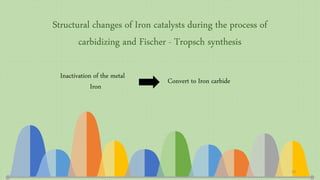
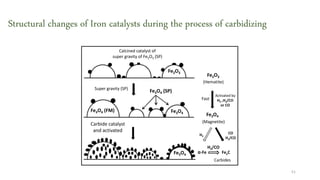
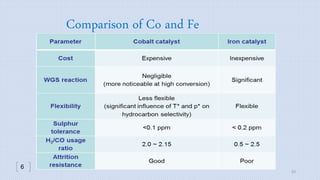
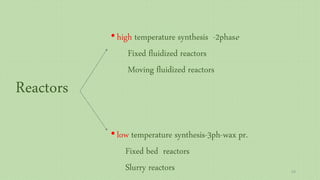
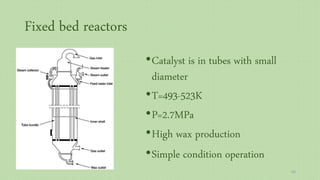
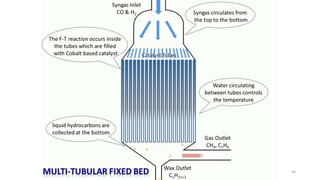
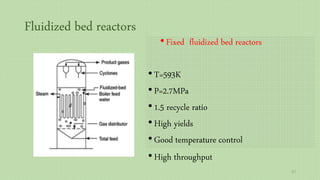
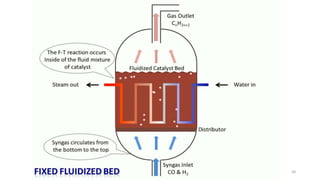
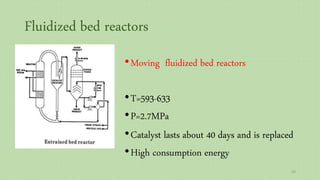
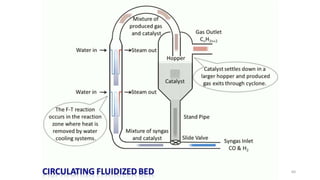
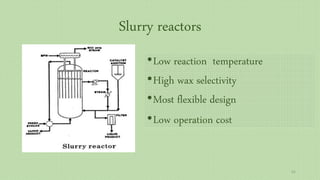
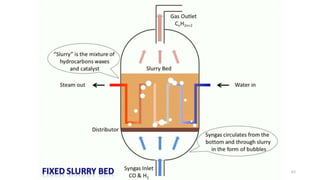
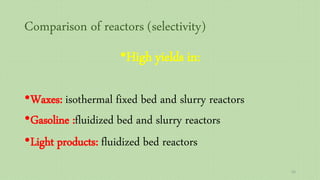
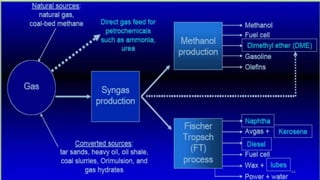
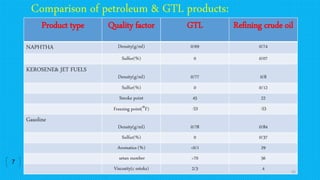
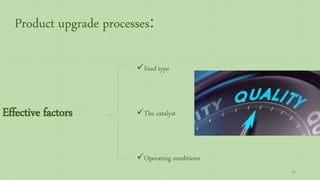
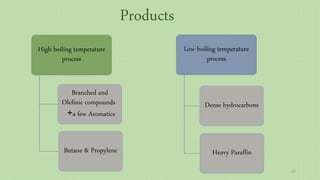
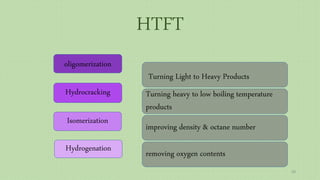
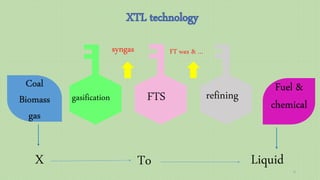
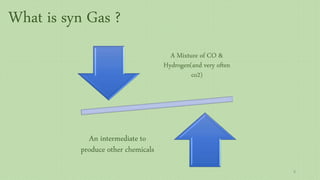
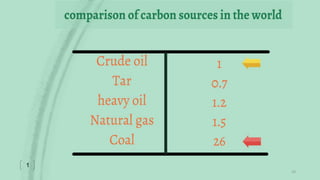
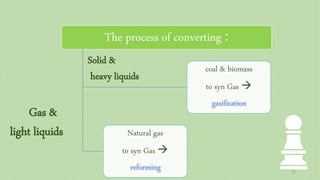
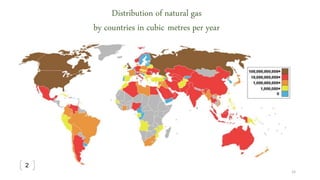
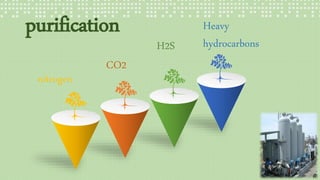
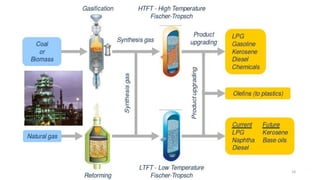
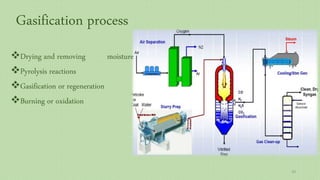
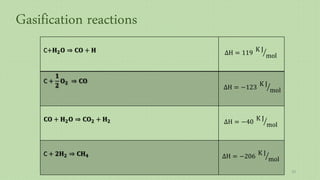
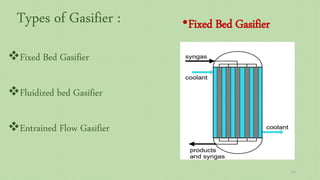
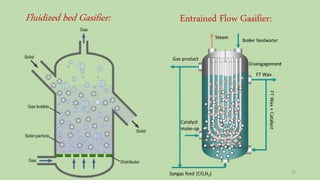
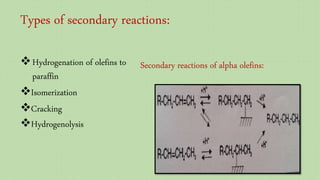
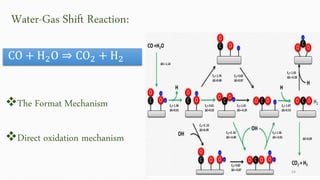
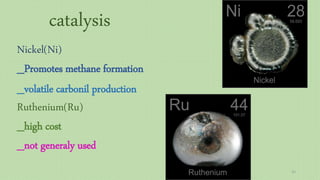
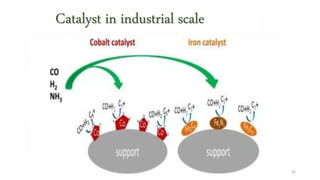
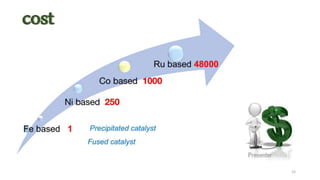
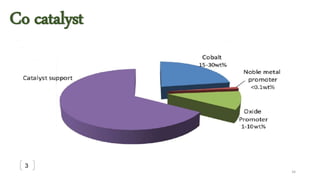
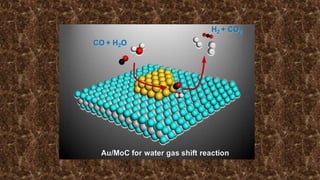
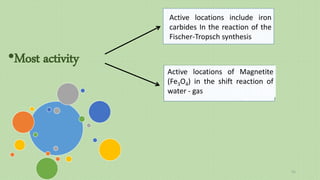
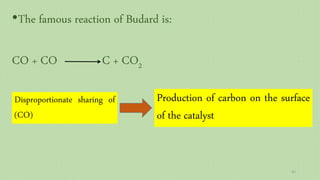
This document provides an overview of Fischer-Tropsch synthesis technology for producing synthetic fuels and chemicals from natural gas and other resources. It discusses the key steps which include gasification of resources to produce syngas, reforming of natural gas to syngas, water-gas shift reaction, and the Fischer-Tropsch synthesis over catalysts to produce liquid fuels and waxes. It also summarizes different catalyst types including iron and cobalt catalysts used in the Fischer-Tropsch process and compares various reactor configurations for the synthesis.










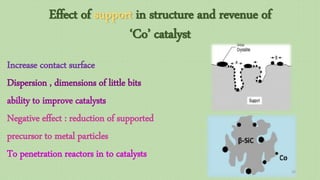
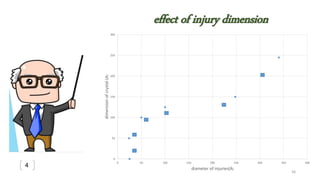

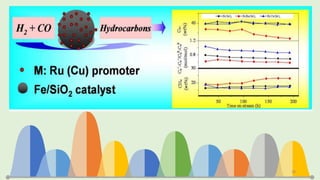
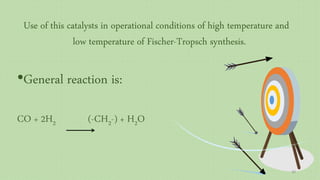
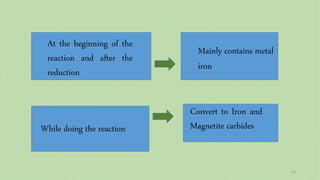
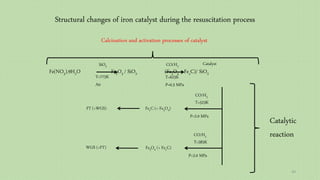
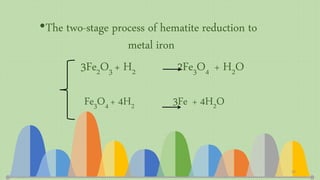
![Evaluation of the possibility of revival hematite to
metal iron
Thermodynamic relationship of
the revival process ΔG = nRT ln[(PH2O/ PH2)/(PH2O/ PH2)eq]
46](https://image.slidesharecdn.com/fischertropsch-180918115832/85/Fischer-tropsch-46-320.jpg)