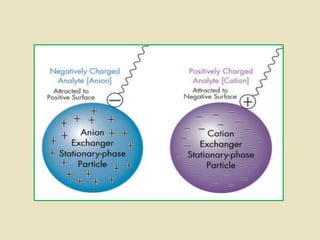
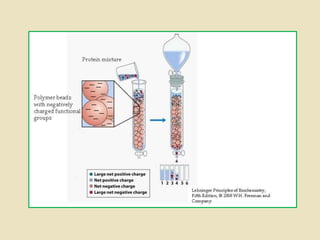
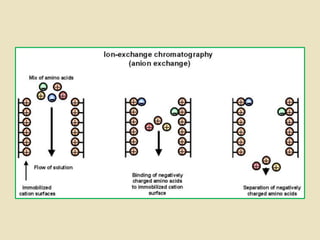
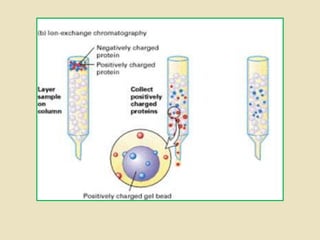
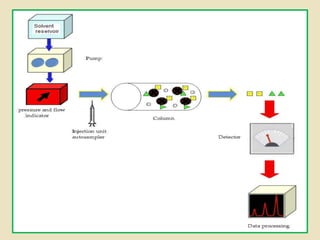
Ion exchange chromatography is a powerful separation technique that separates components based on their charge. It involves using a stationary phase with charged groups that interact with the ionized groups on molecules in the mobile phase. The molecules are separated from a mixture as they compete for binding sites on the oppositely charged stationary phase. Common applications include purifying proteins, peptides, enzymes, and other biomolecules.