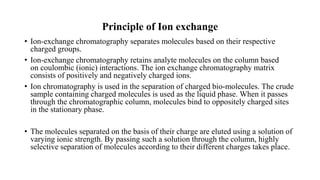
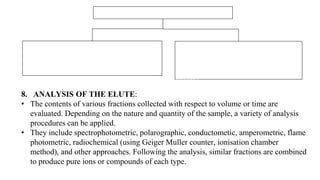
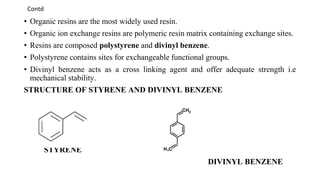
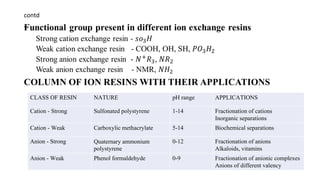
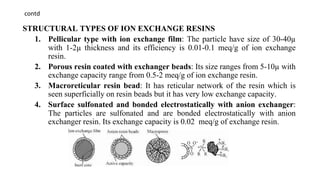
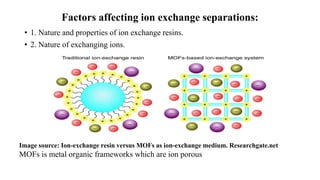
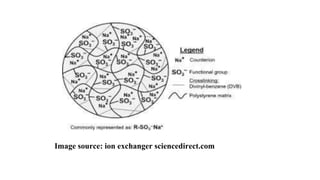
The document discusses ion exchange chromatography, including its principle of separating ions and polar molecules based on their charge affinity for the ion exchanger. It covers various aspects of ion exchange chromatography such as classifications of resins, factors affecting separations, practical requirements like column materials and dimensions, and applications in areas like biochemistry, inorganic separations, and water analysis.