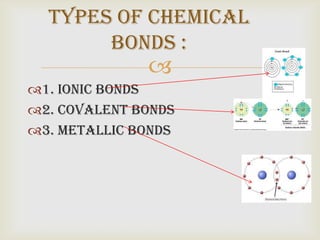
This document discusses different types of chemical bonds: ionic bonds form when atoms gain or lose valence electrons to become ions with opposite charges that attract. Covalent bonds form when two atoms share valence electrons in single, double or triple bonds. Metallic bonds are characteristic of metals, with mobile valence electrons shared throughout the crystal structure. Ionic compounds are solid, often water-soluble with high melting/boiling points and low thermal conductivity.