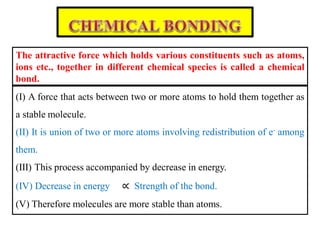
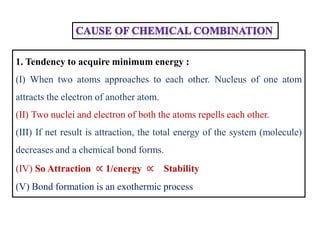
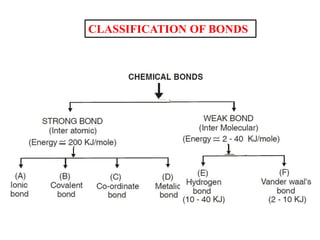
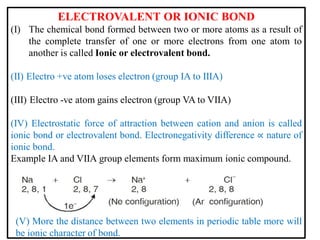
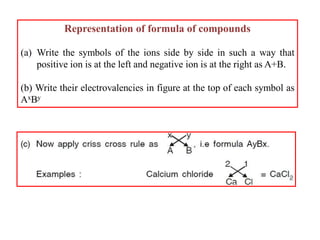
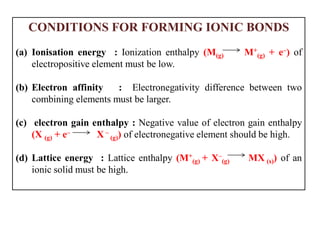
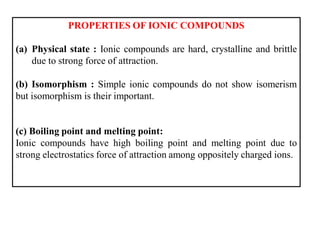
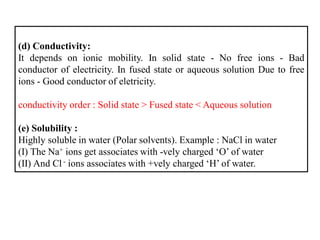
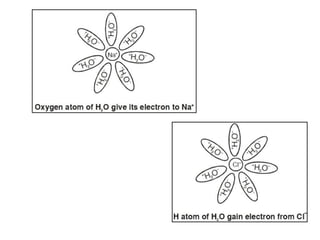
A chemical bond is the attractive force that holds atoms or ions together in a molecule or crystalline solid. Ionic or electrovalent bonds form when electrons are transferred from one atom to another, resulting in cations and anions. The electrostatic force of attraction between oppositely charged ions is the ionic bond. Ionic compounds have high melting and boiling points due to the strong electrostatic attractions between ions. They are typically solids and are good conductors of electricity when molten or dissolved due to free ions.