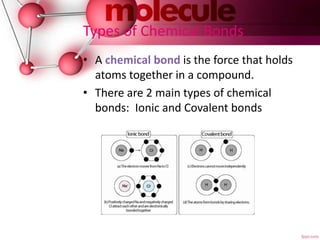
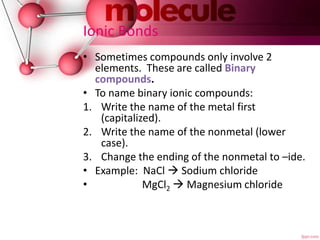
This document defines chemical formulas and explains how atoms form compounds. It states that a chemical formula tells what elements are in a compound and the number of atoms. It then discusses that atoms form compounds due to electric forces between protons and electrons. Atoms seek to gain or lose electrons to achieve a stable outer electron level. The document goes on to describe ionic and covalent bonds. Ionic bonds form when a metal transfers electrons to a nonmetal. Covalent bonds form when nonmetals share electrons. It provides examples of naming ionic and covalent compounds.