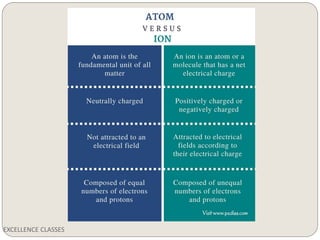
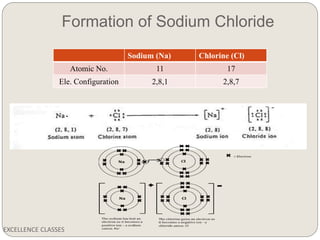
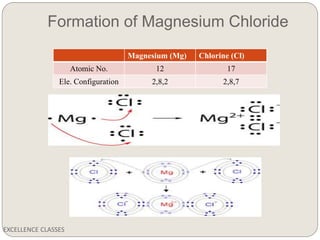
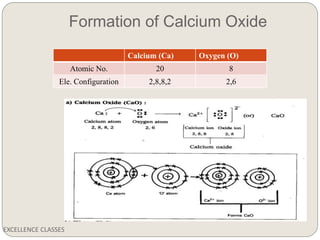
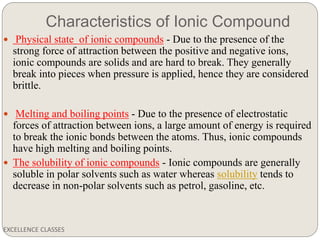
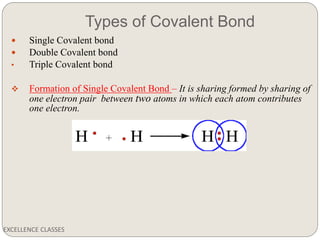
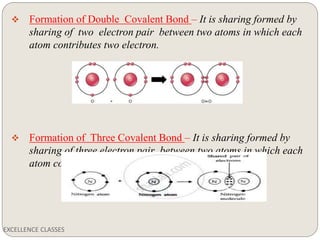
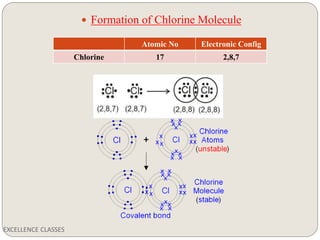
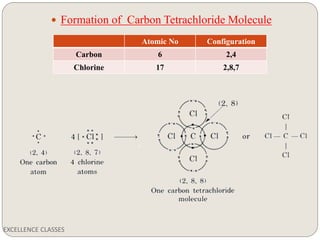
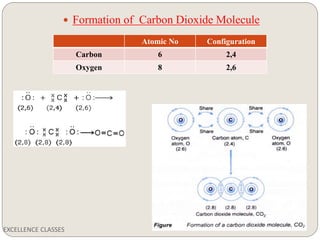
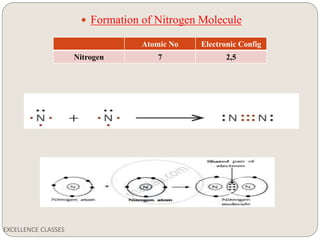
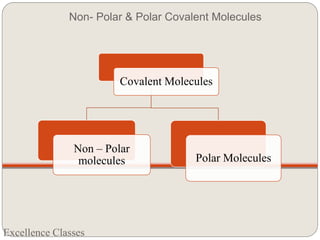
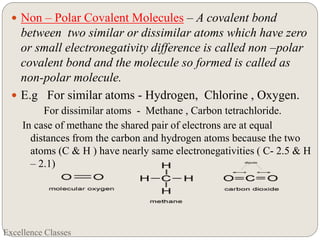
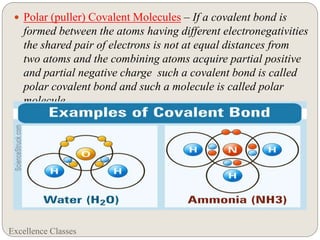
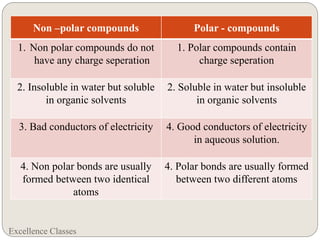
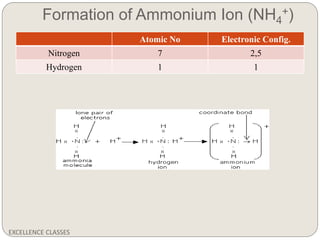
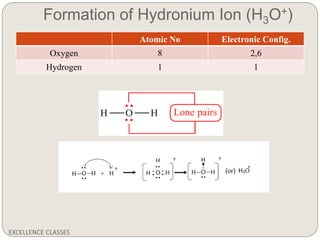
The document provides an introduction to chemical bonding, including definitions of key terms like chemical bond, ionic bond, covalent bond, and coordinate bond. It describes the three main types of bonds: ionic formed by electron transfer, covalent formed by electron sharing, and coordinate bonds formed when one atom provides both electrons. Examples of bond formation are given for ionic compounds like NaCl and MgCl2 and covalent compounds like Cl2, CO2, and NH3. Characteristics of ionic and covalent compounds are also summarized.