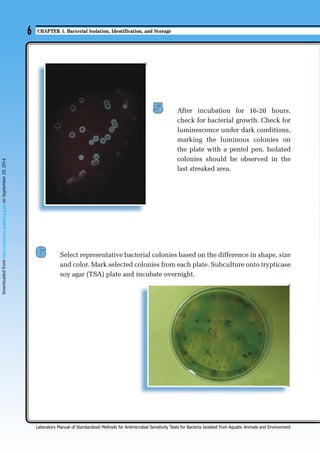
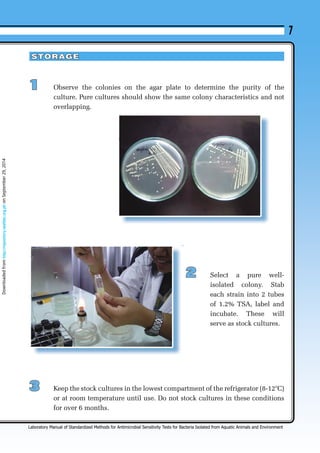
This chapter describes the process of bacterial isolation, identification, and storage. Key steps include:
1. Isolating bacteria from fish and shrimp tissues using streak plating to obtain pure cultures for further analysis.
2. Identifying bacteria through a battery of morphological, physiological, and biochemical tests and comparing results to identification schemes.
3. Storing pure bacterial cultures through methods like sub-culturing, refrigeration, freezing, or lyophilization to preserve them for future use. Proper labeling of stored cultures is also emphasized.