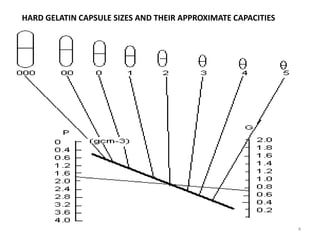
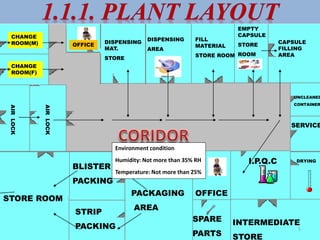
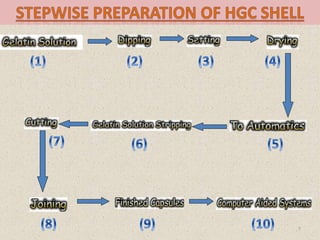
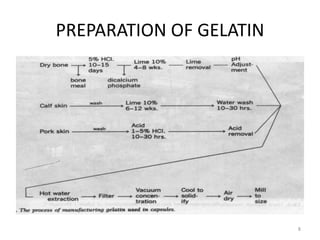
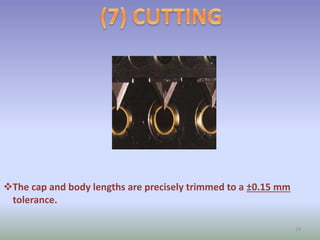
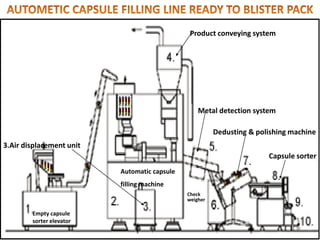
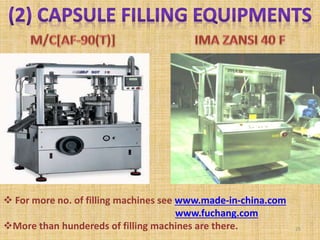
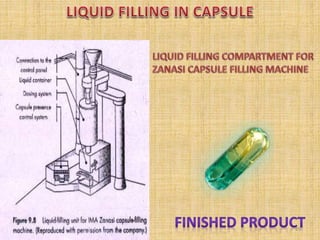
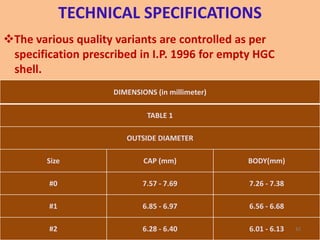
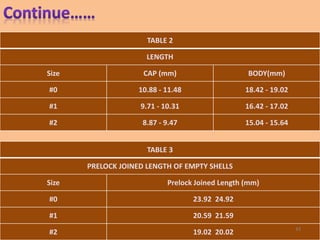
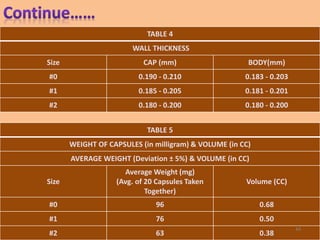
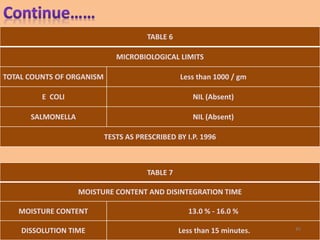
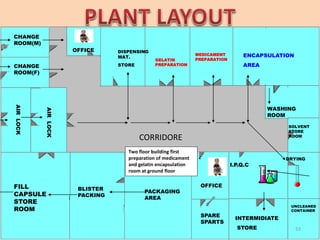
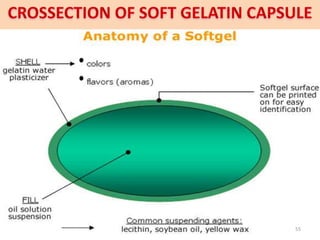
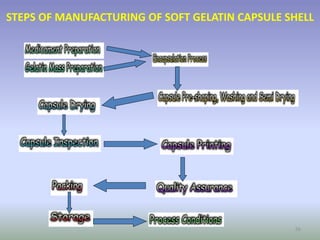
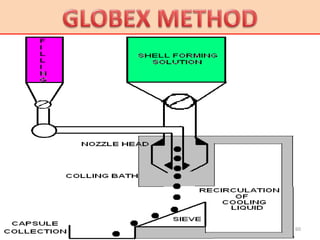
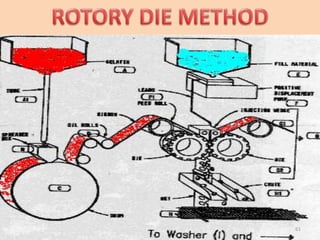
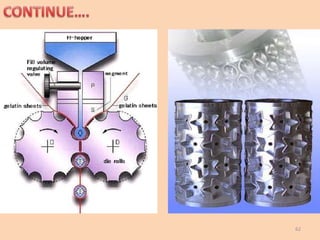
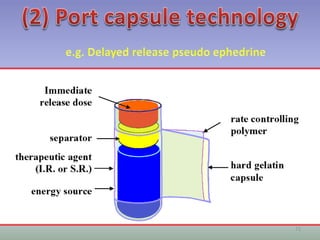
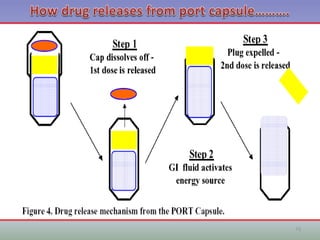
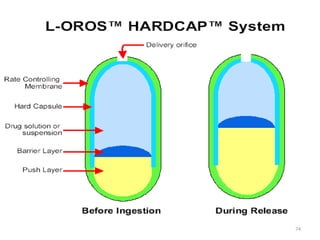
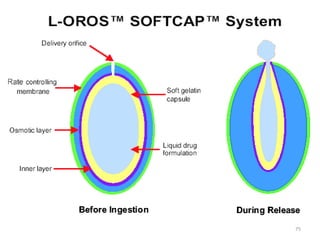
This document provides information on hard gelatin capsules, including their production process, equipment used, quality control tests, and sizes. It discusses the preparation of gelatin, molding capsule halves, drying, trimming, joining, filling, sealing, and packaging processes. Key equipment for filling capsules are also outlined, including elevators, filling machines for powders, granules and liquids, air displacement units, metal detectors, and sorting machines. Standard operating procedures and environmental conditions for capsule filling are also provided.