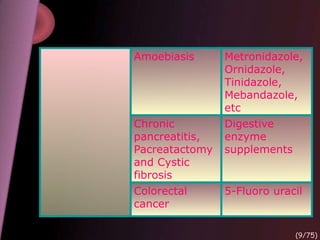
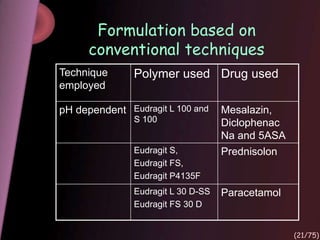
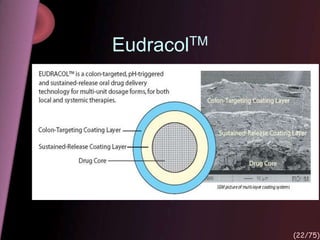
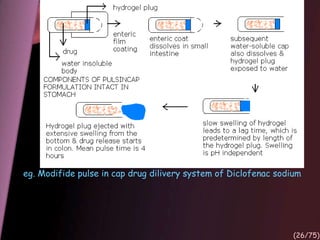
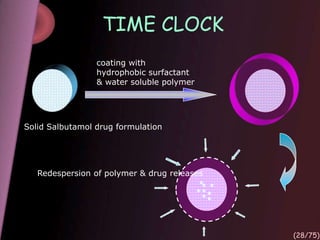
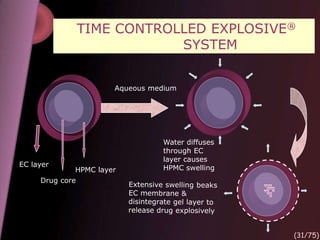
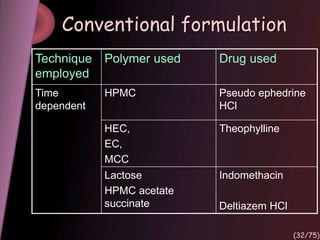
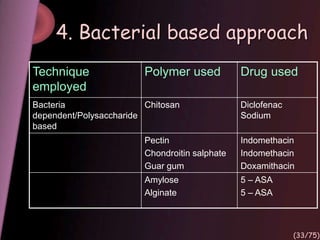
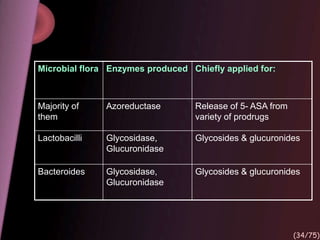
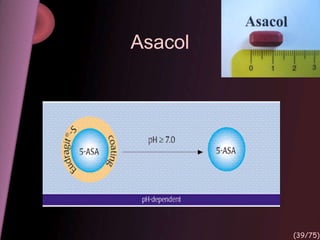
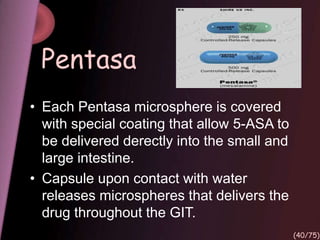
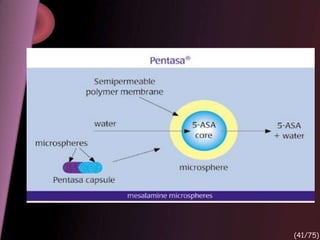
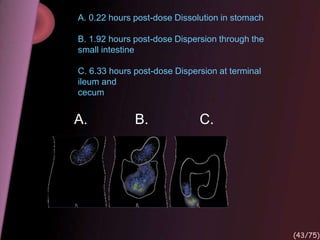
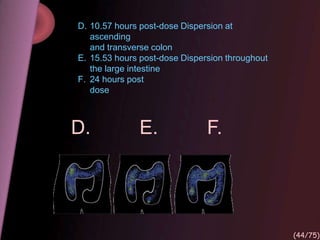
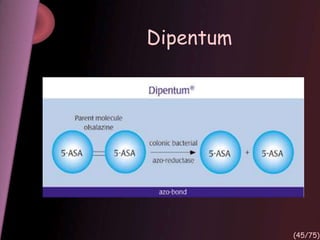
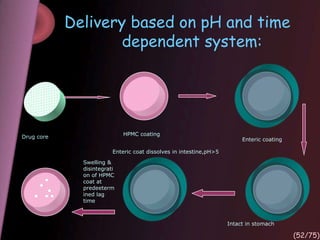
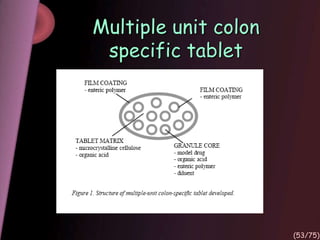
This document provides an overview of colon targeted drug delivery systems. It discusses the anatomy of the colon, challenges in delivering drugs to the colon, and various pharmaceutical approaches for colon targeted delivery including pH dependent systems, time dependent systems, microflora activated systems, and multiparticulate systems. Several market formulations that use these approaches are also summarized, including Pentasa, Dipentum, Colazal, and Egalet.