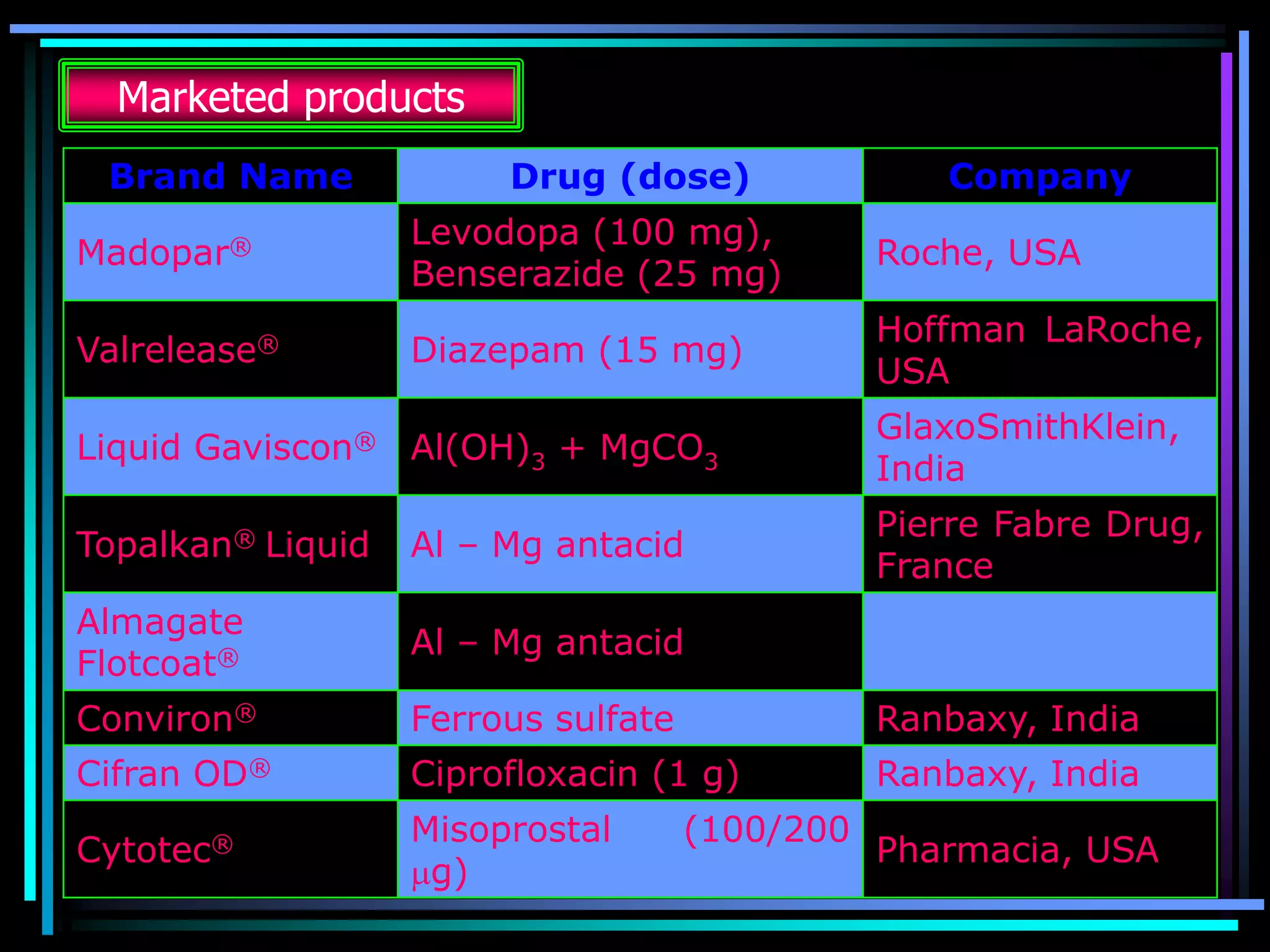
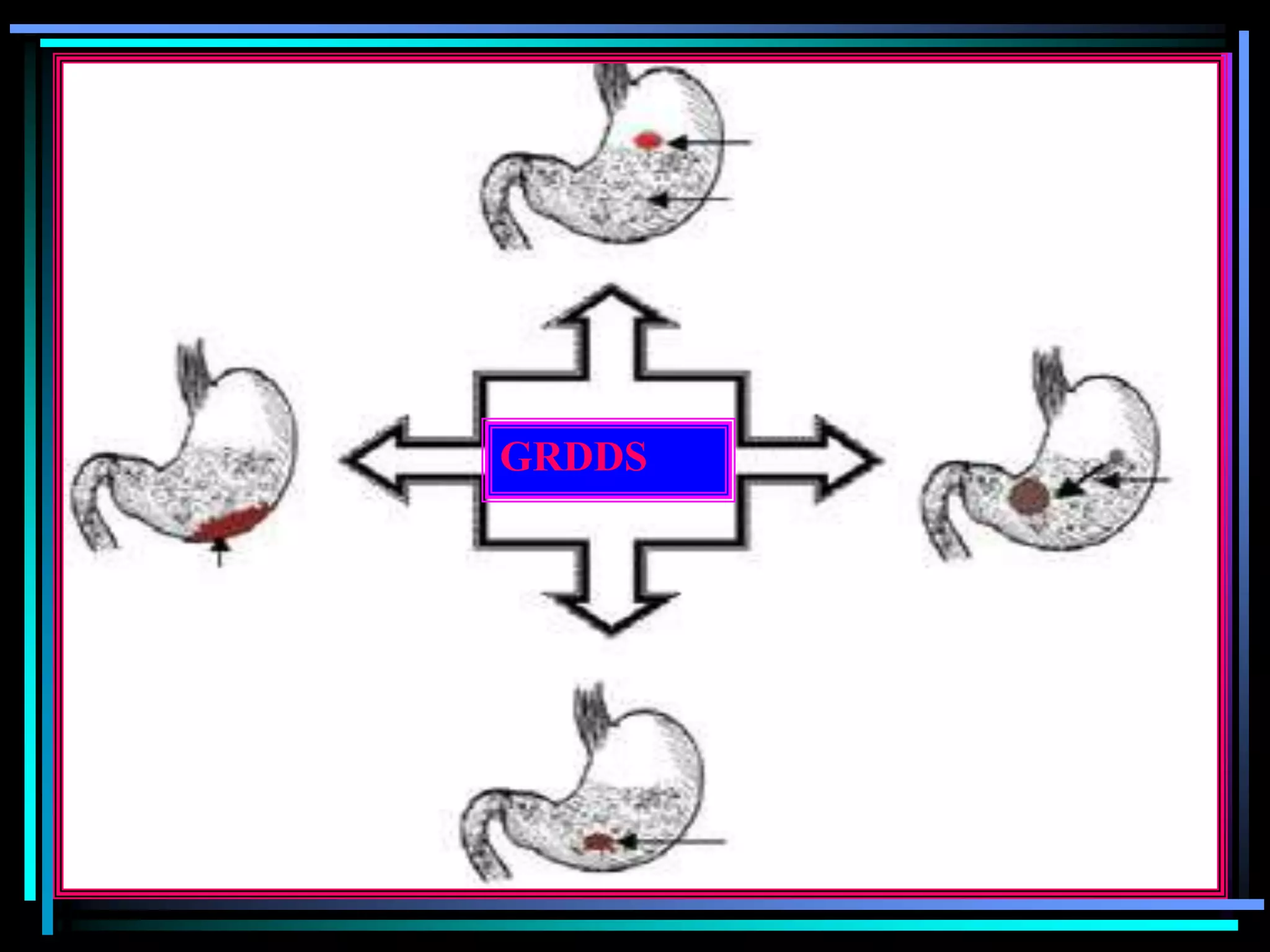
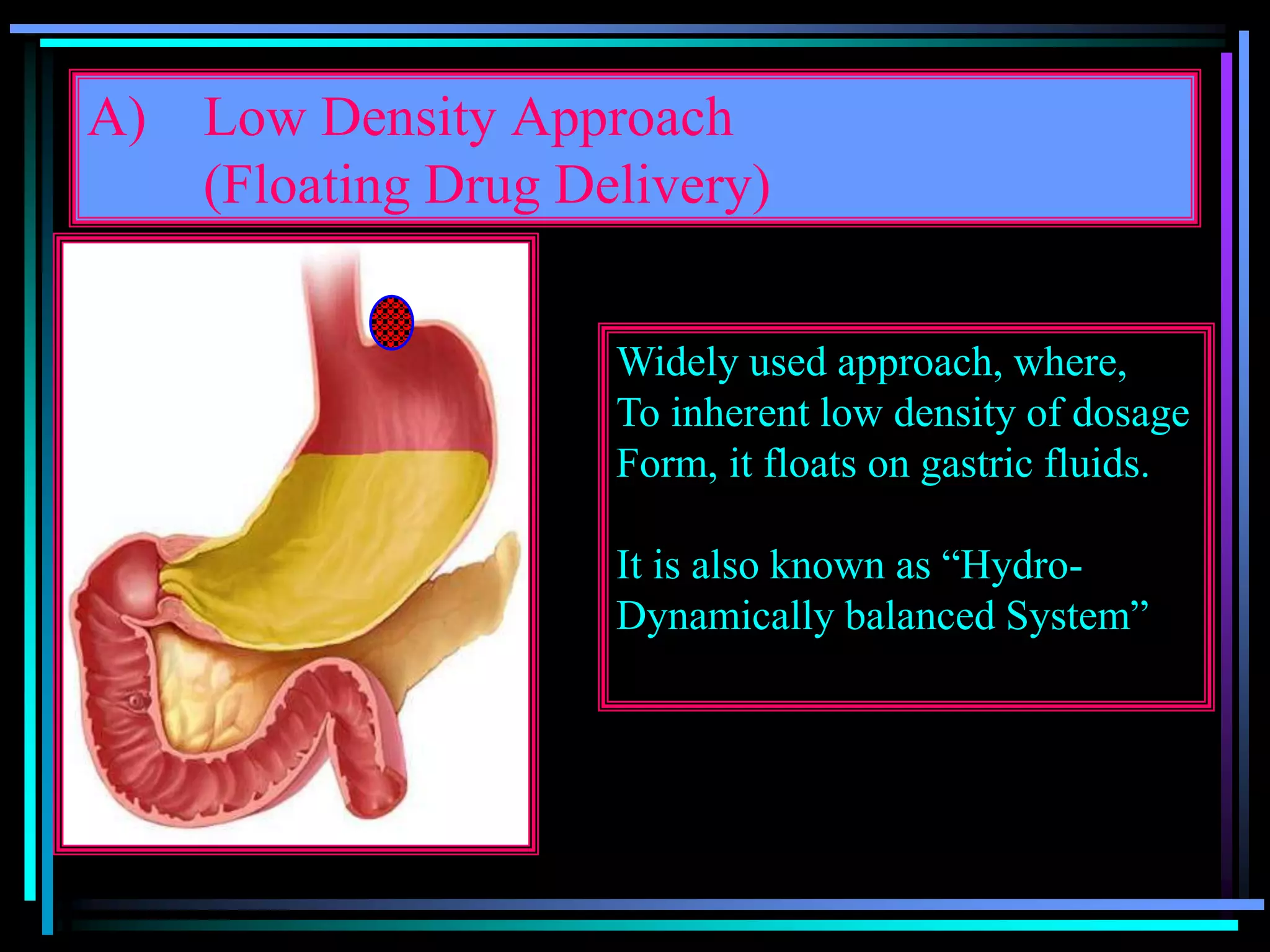
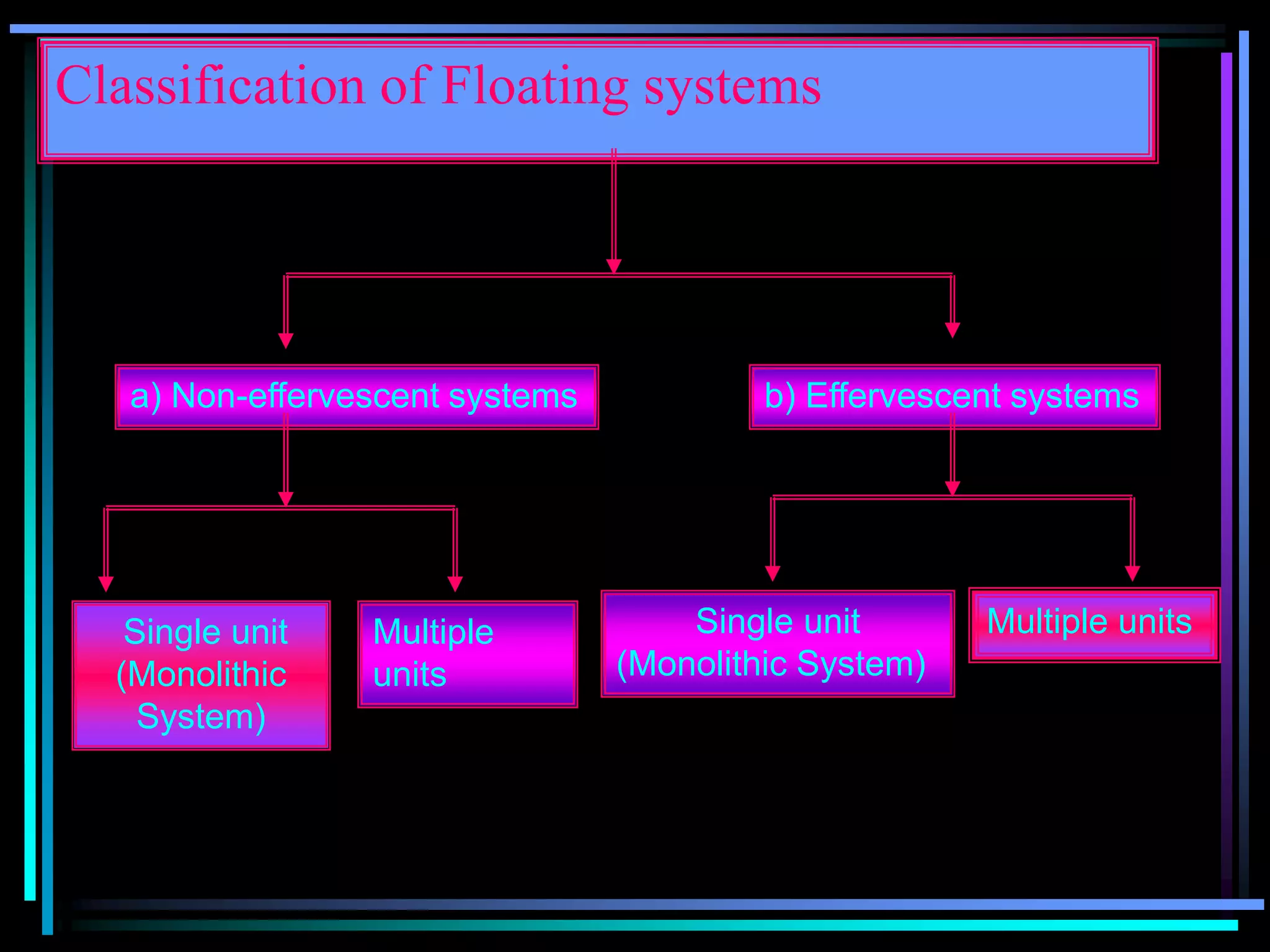
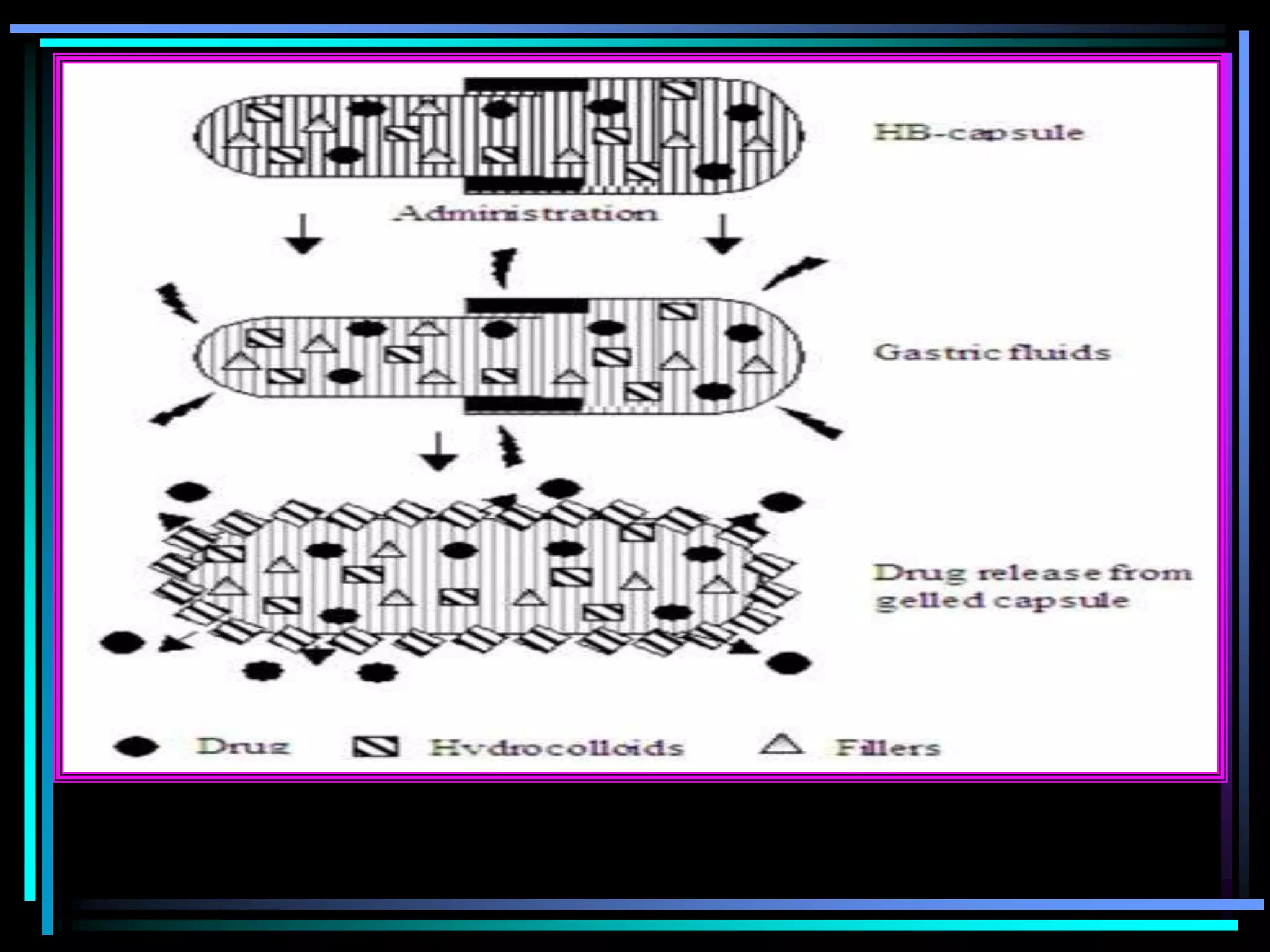
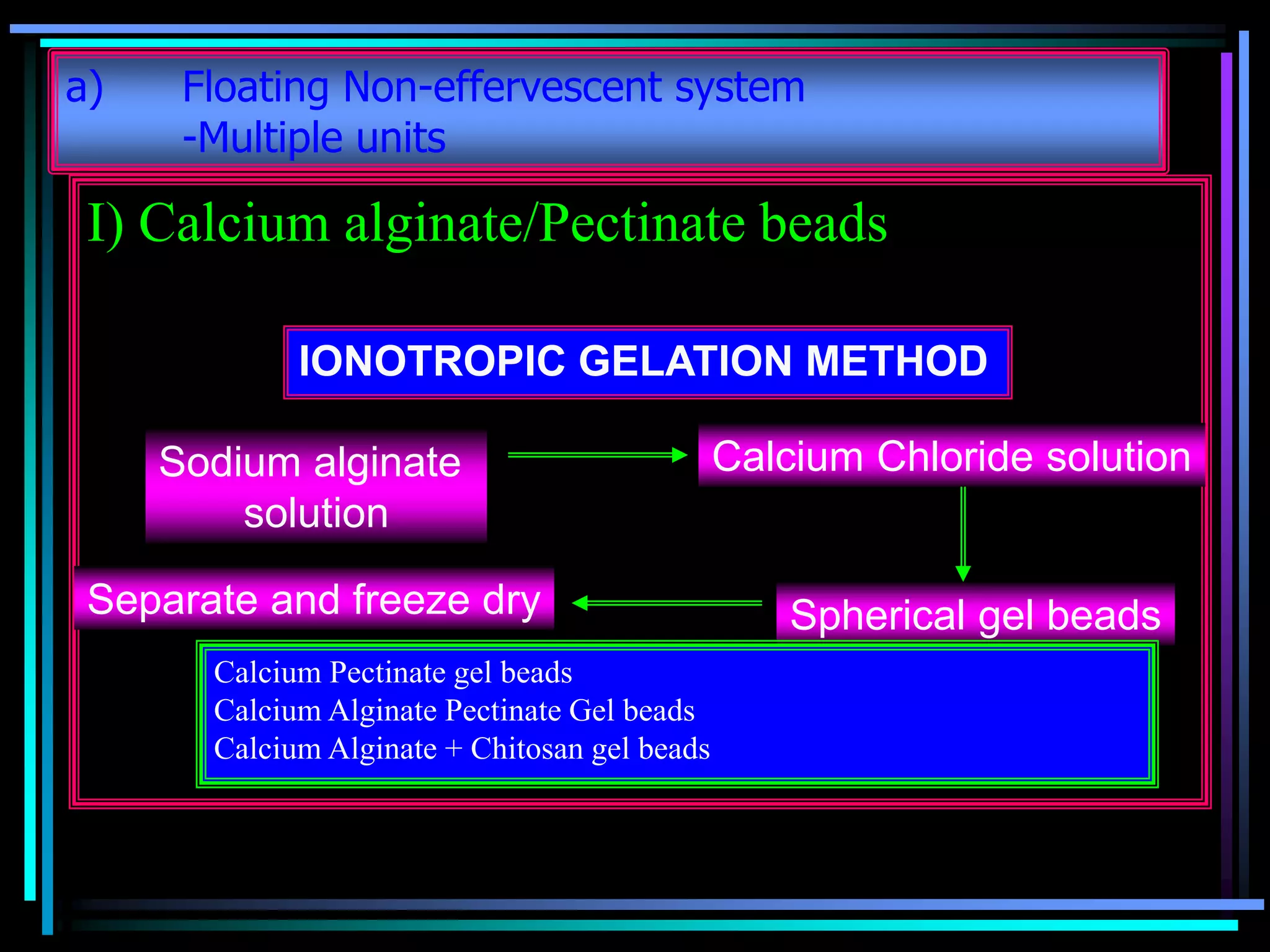
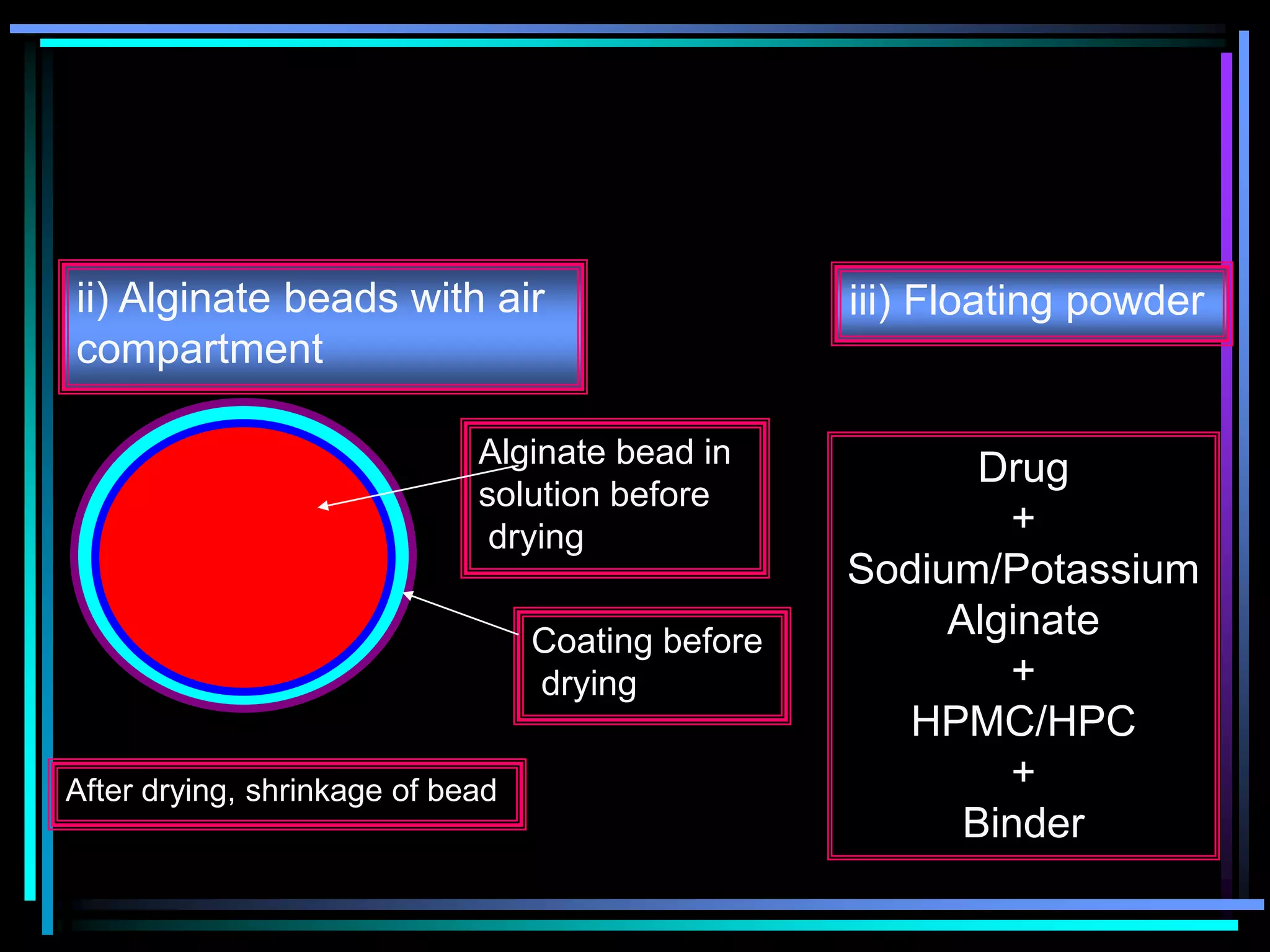
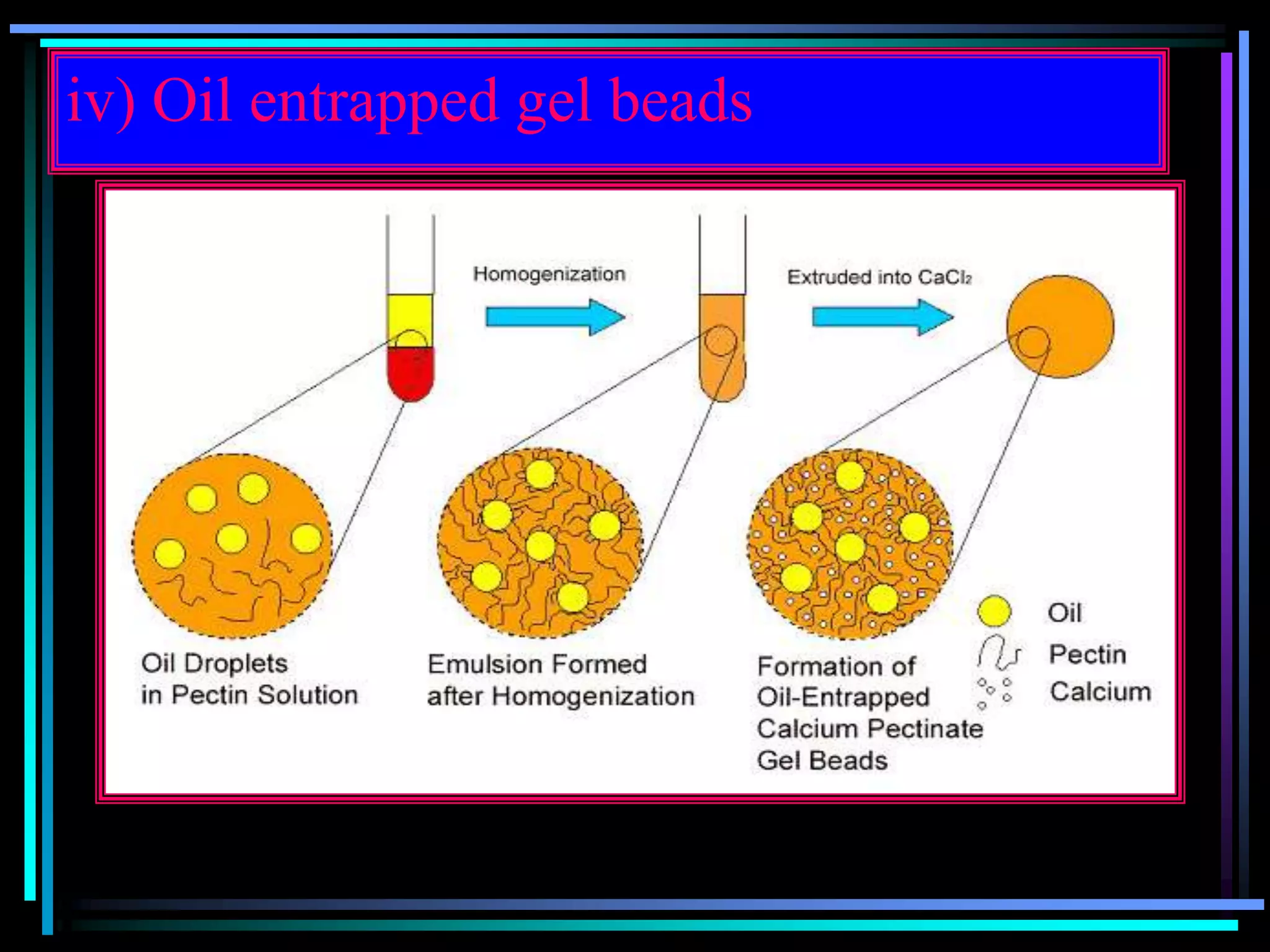
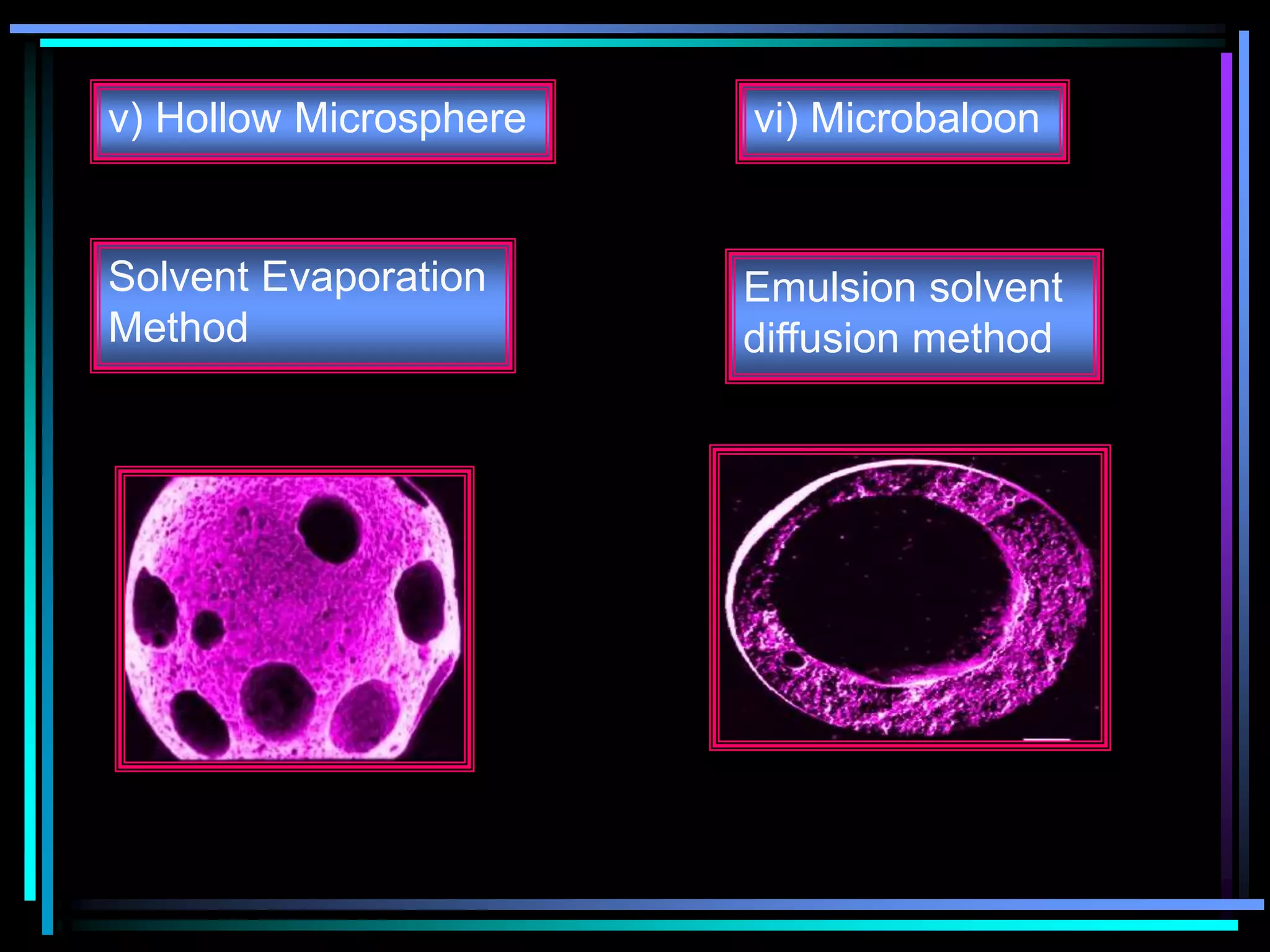
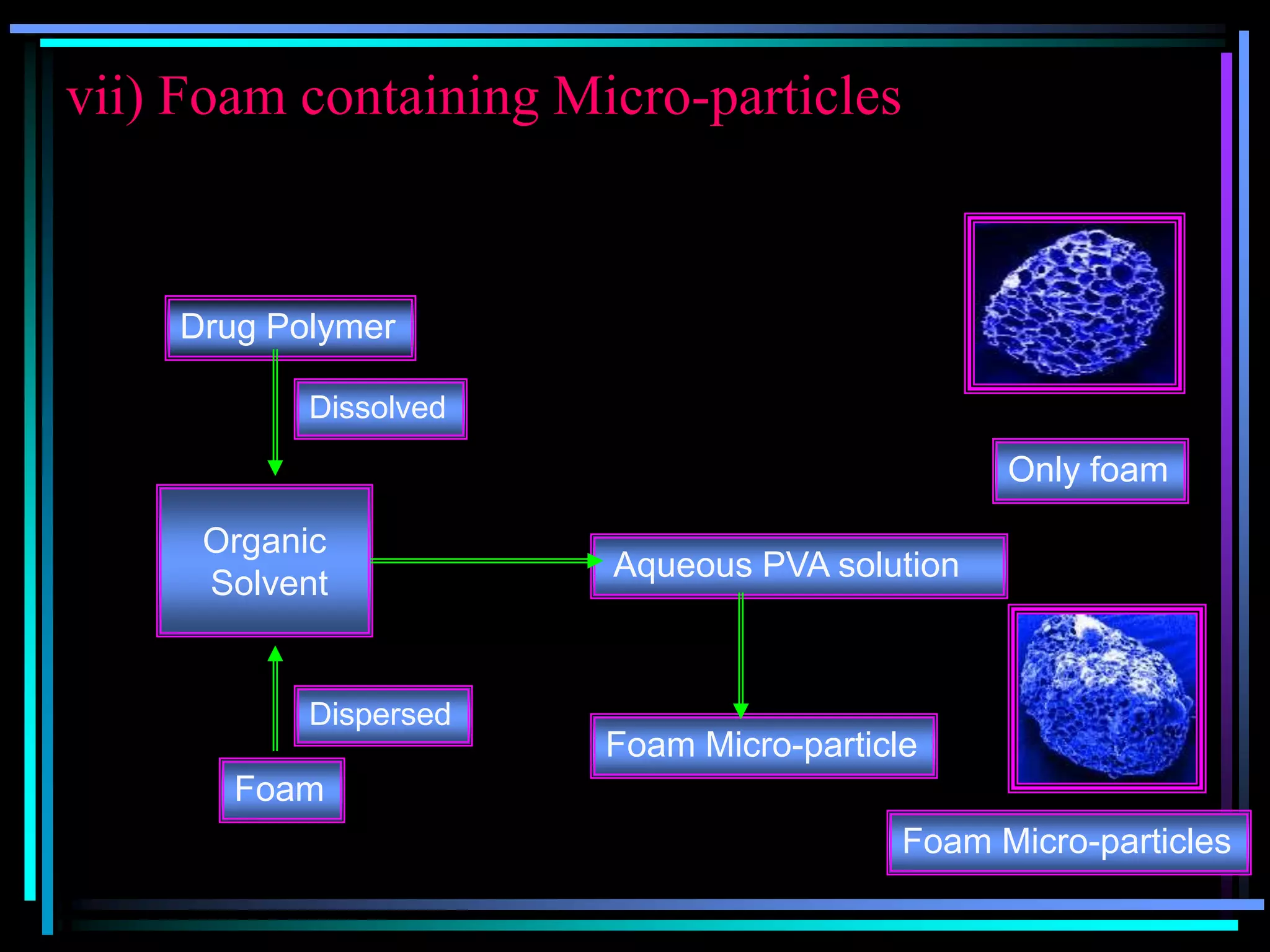
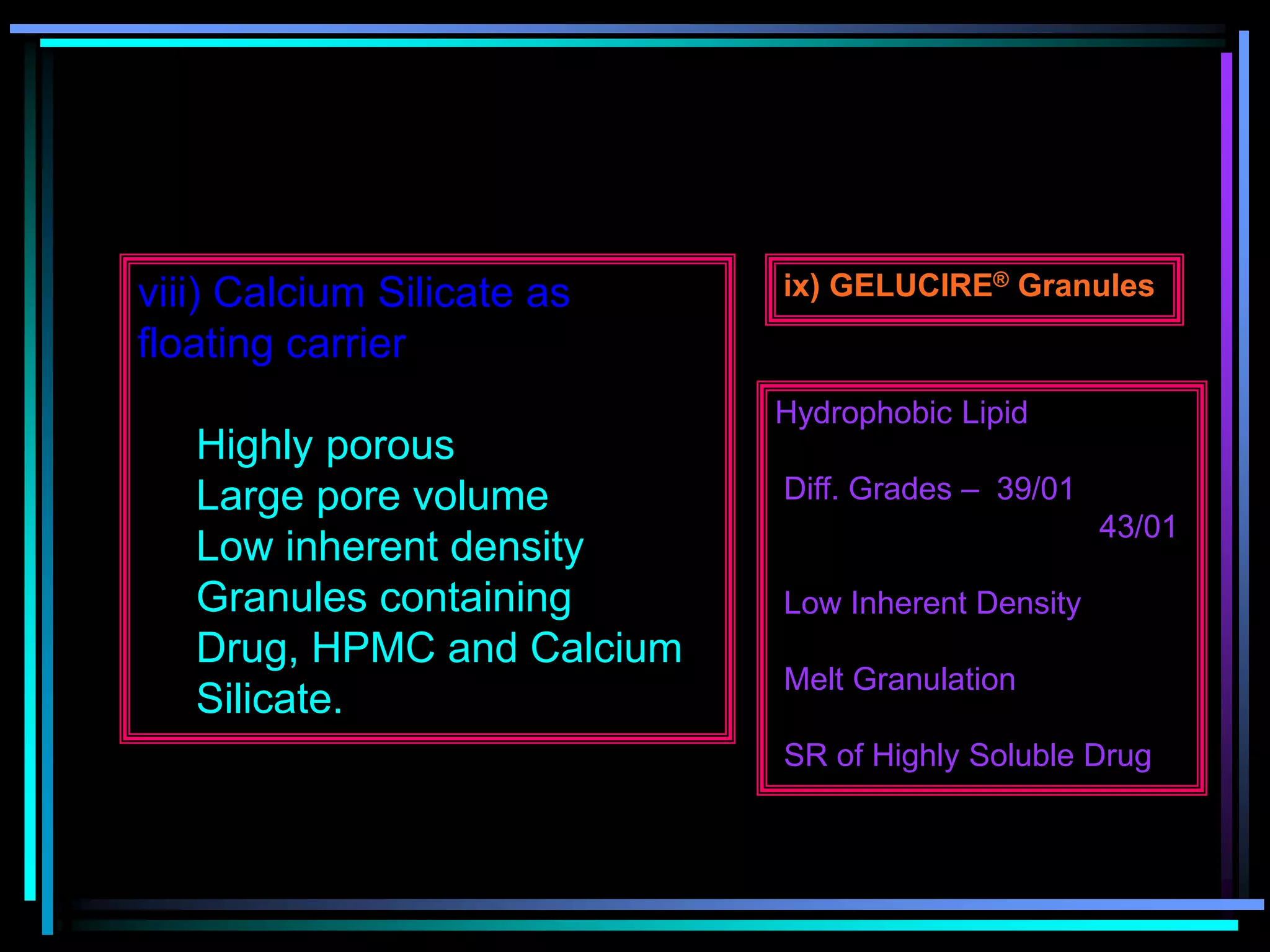
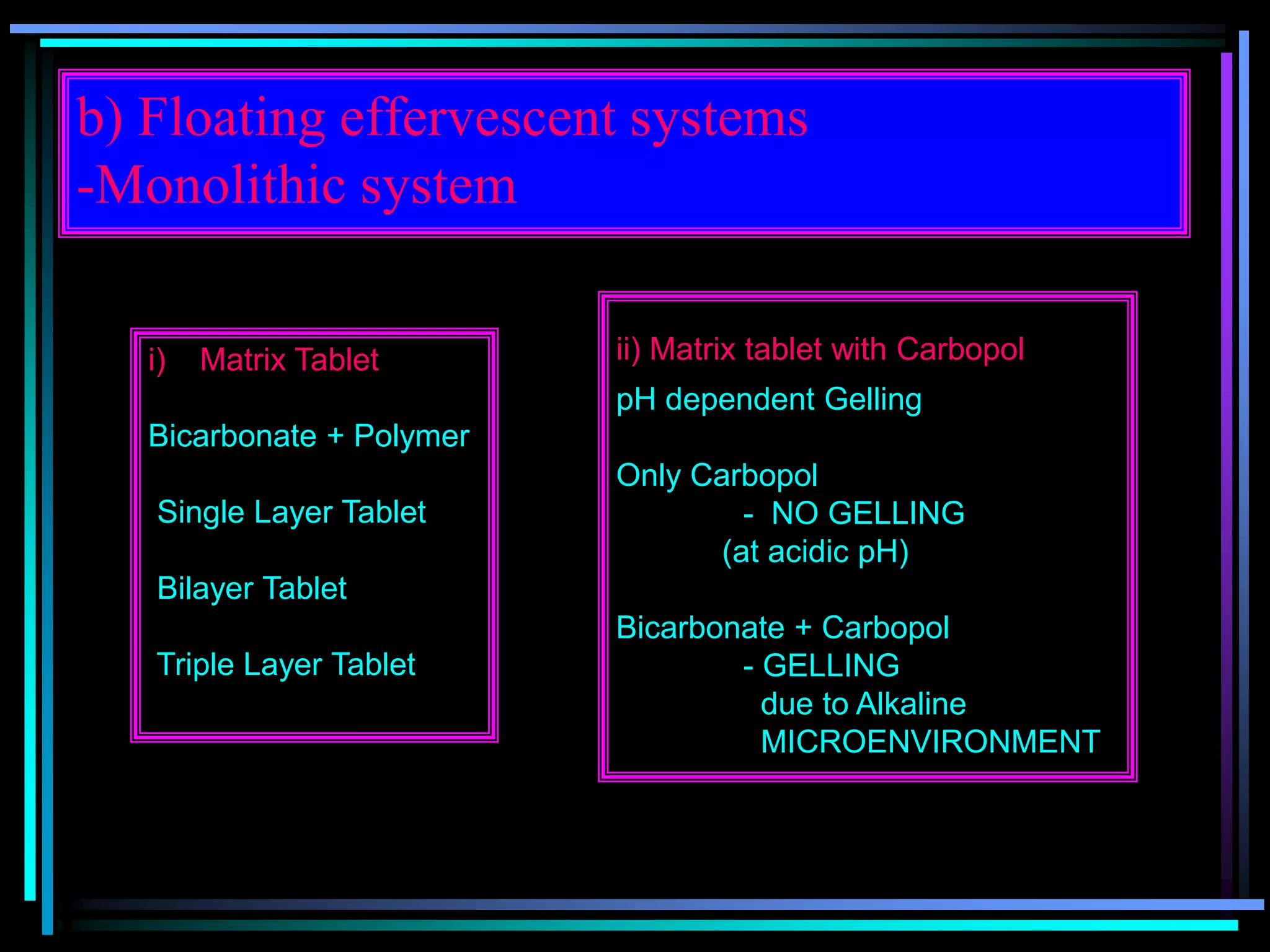
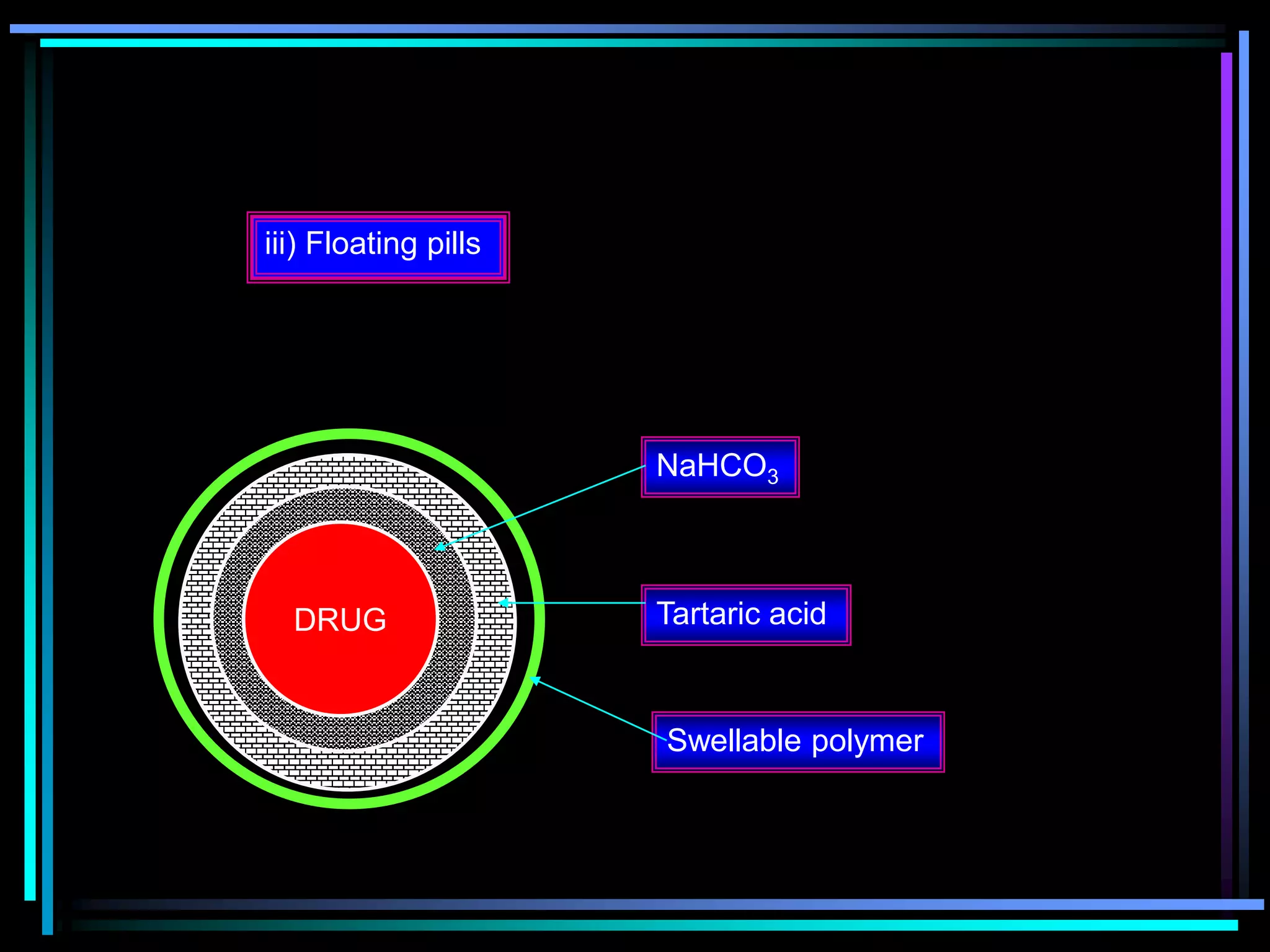
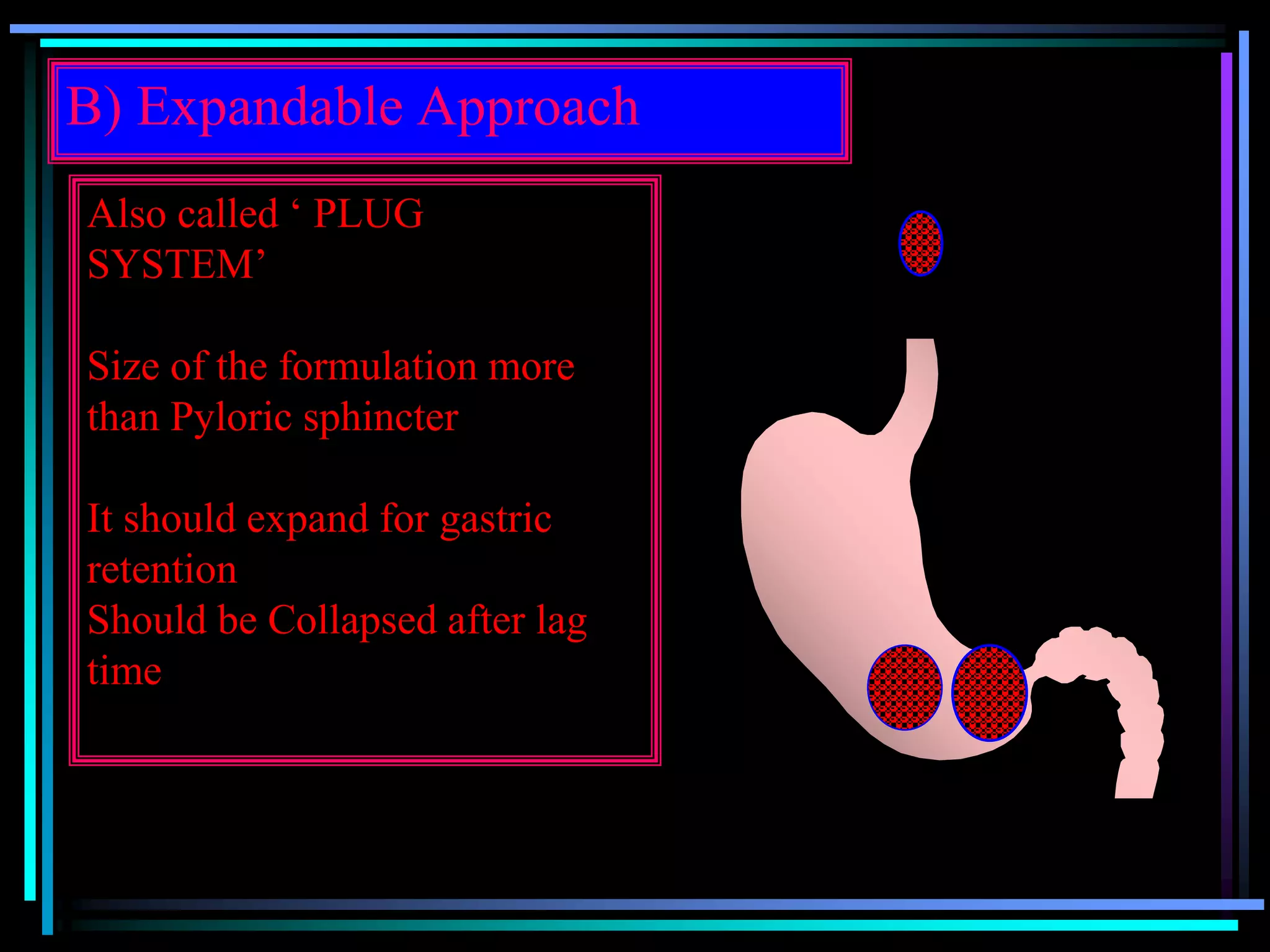
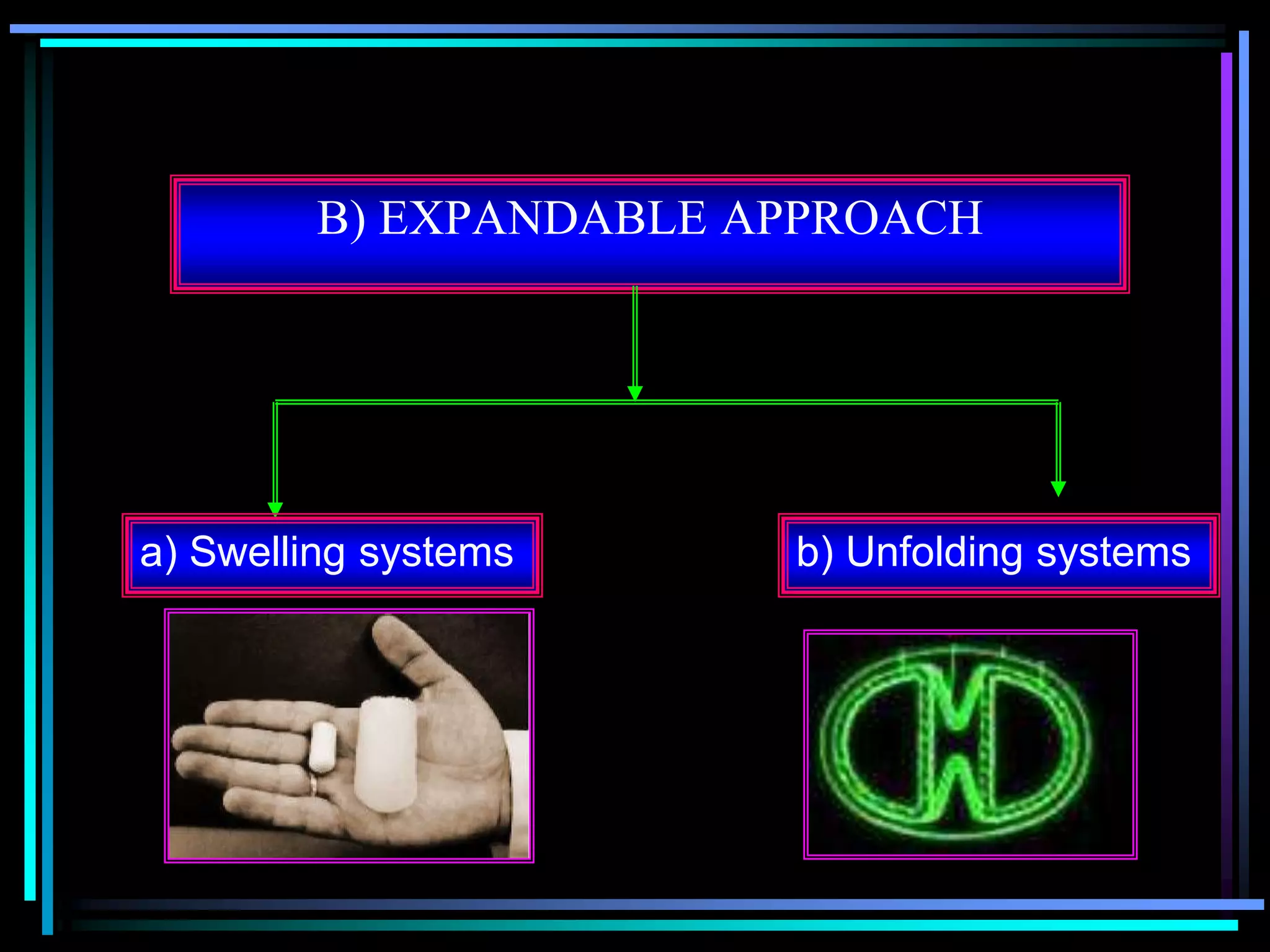
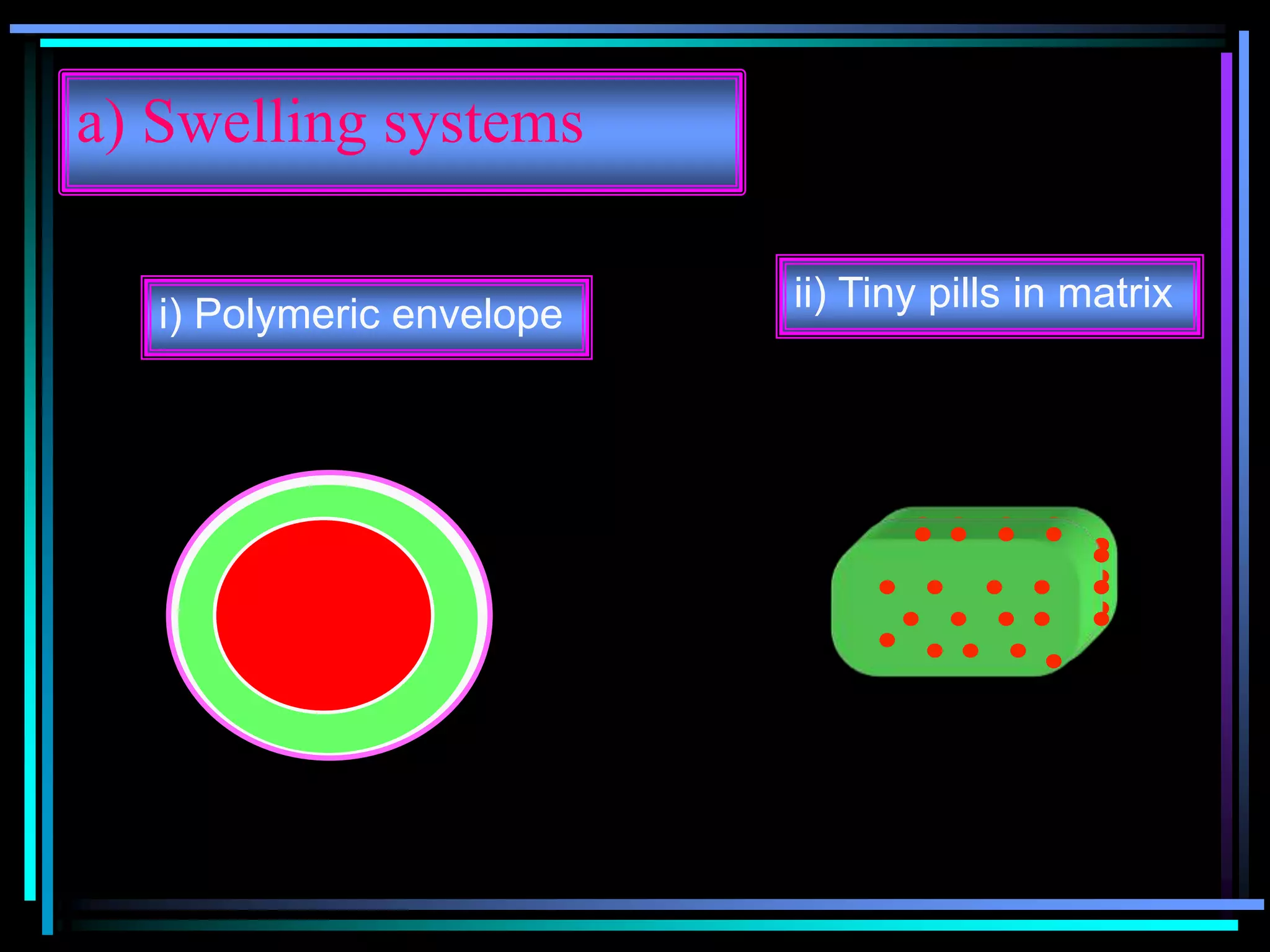
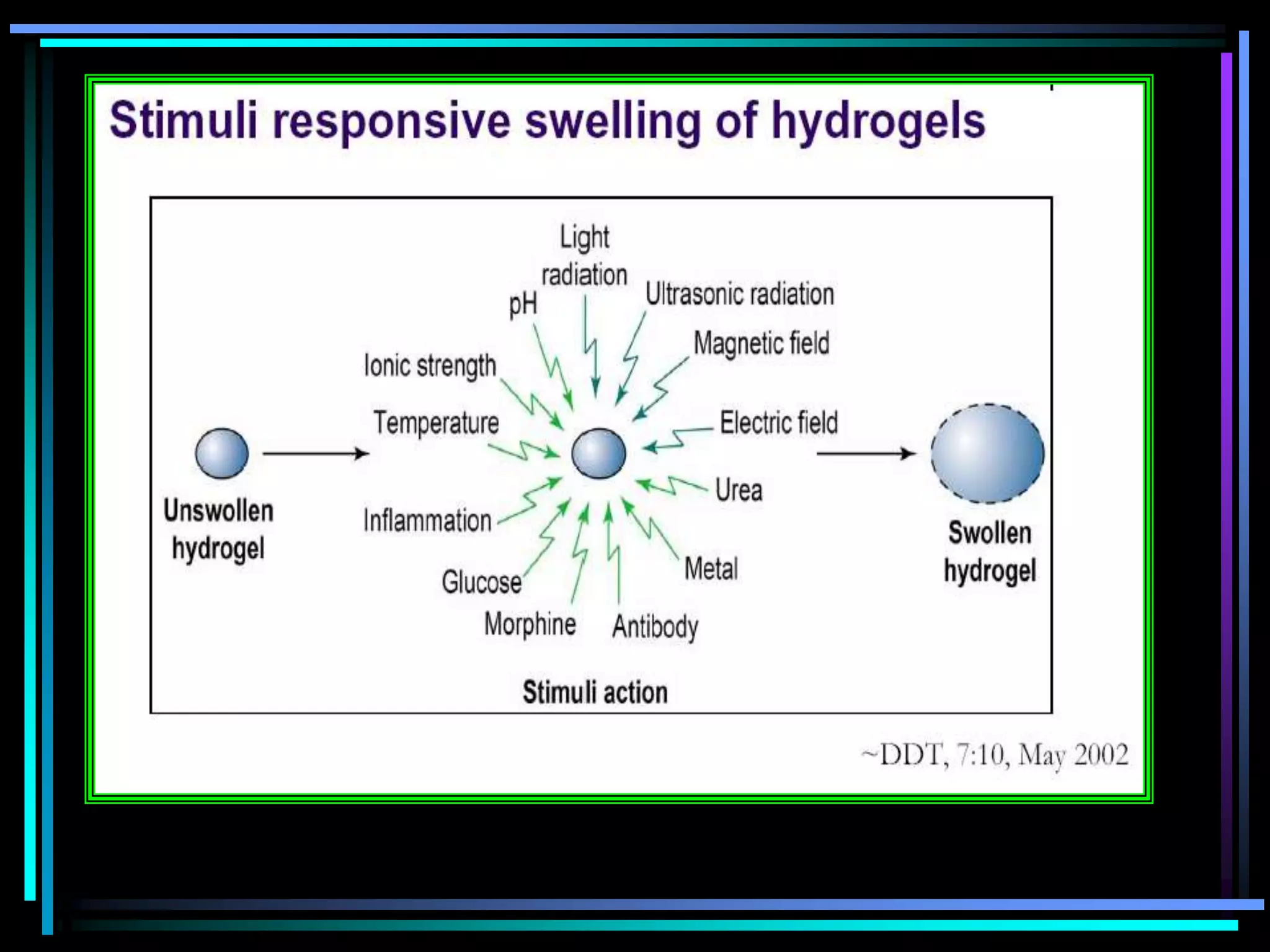
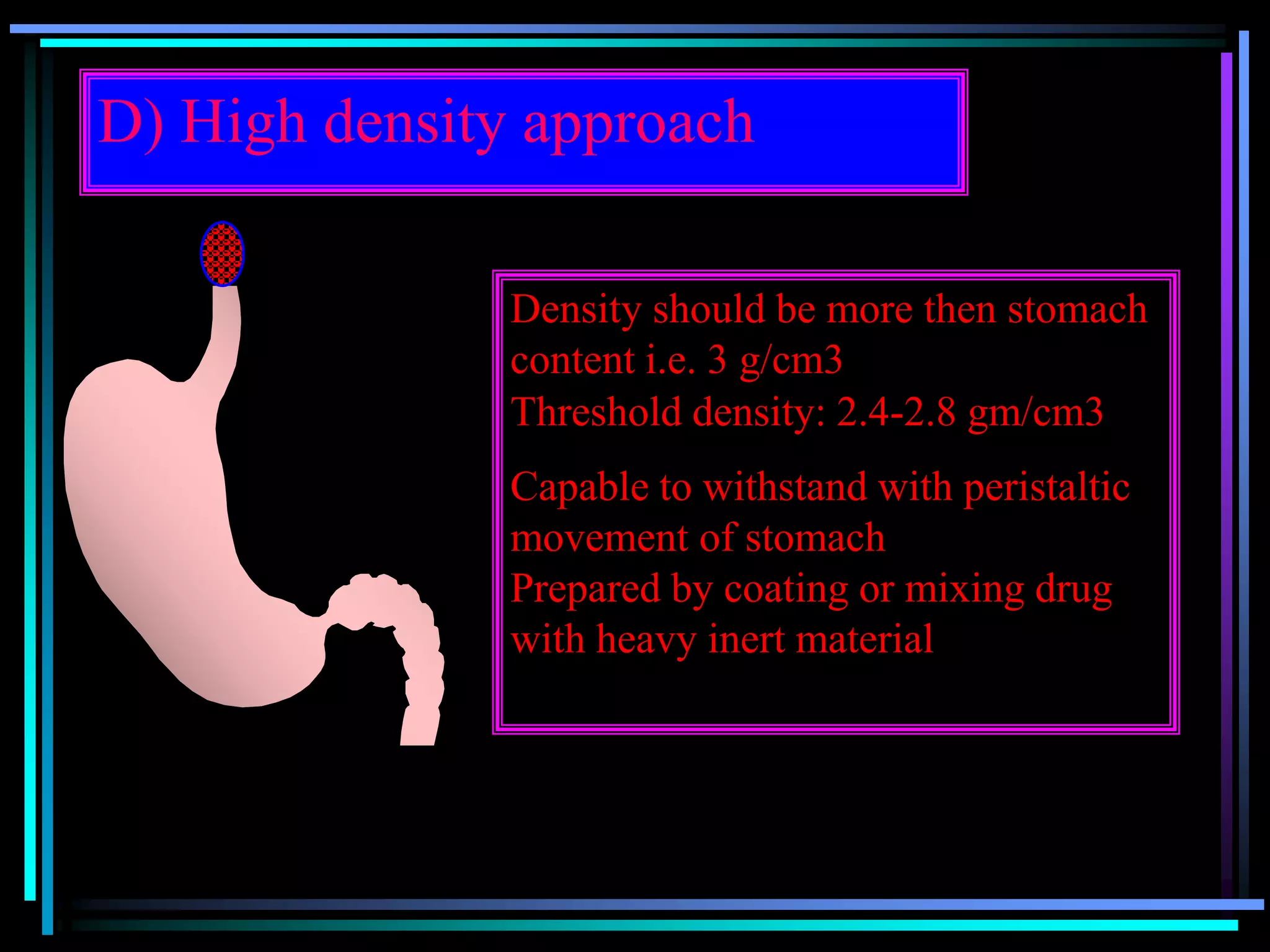
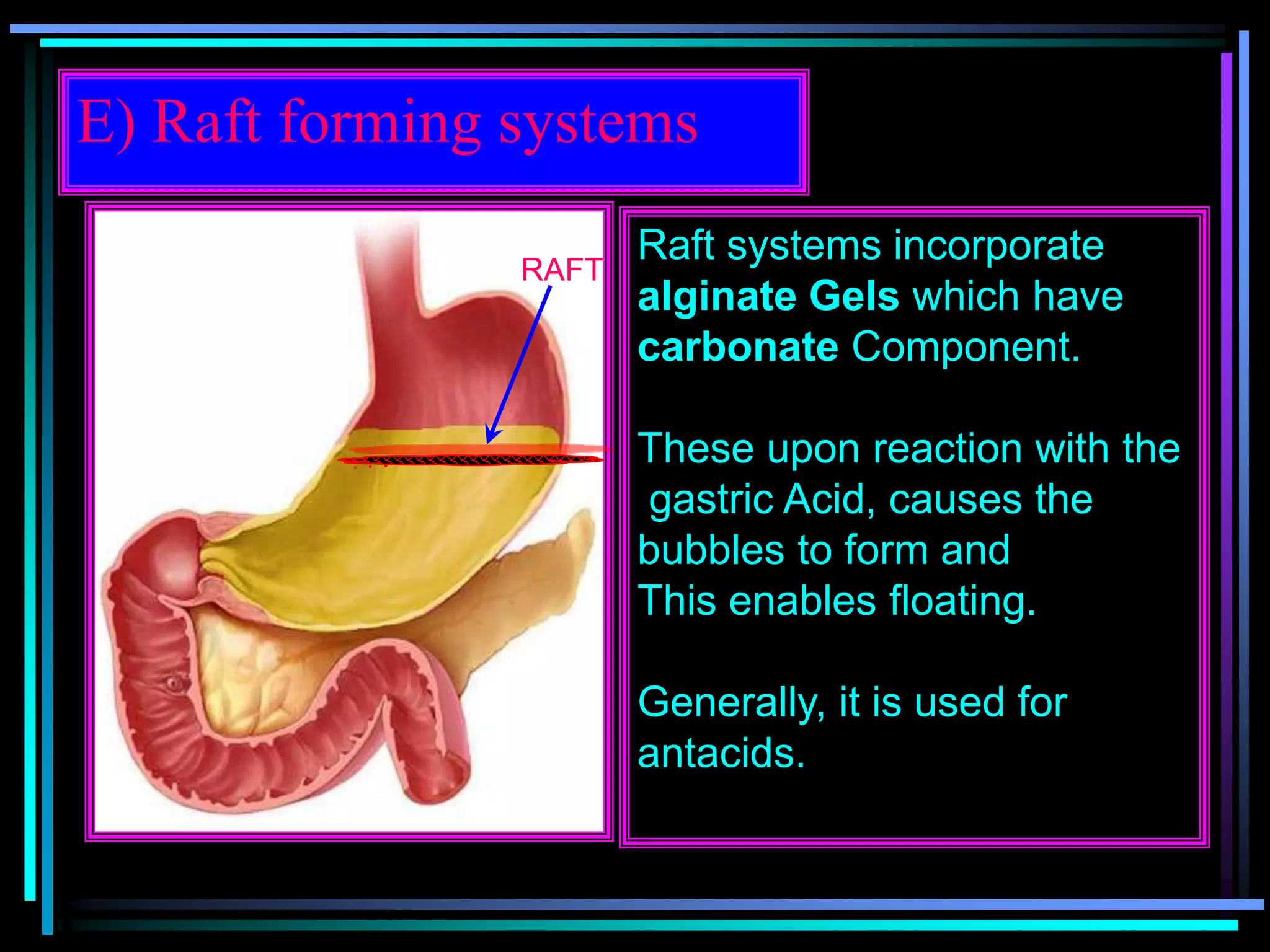
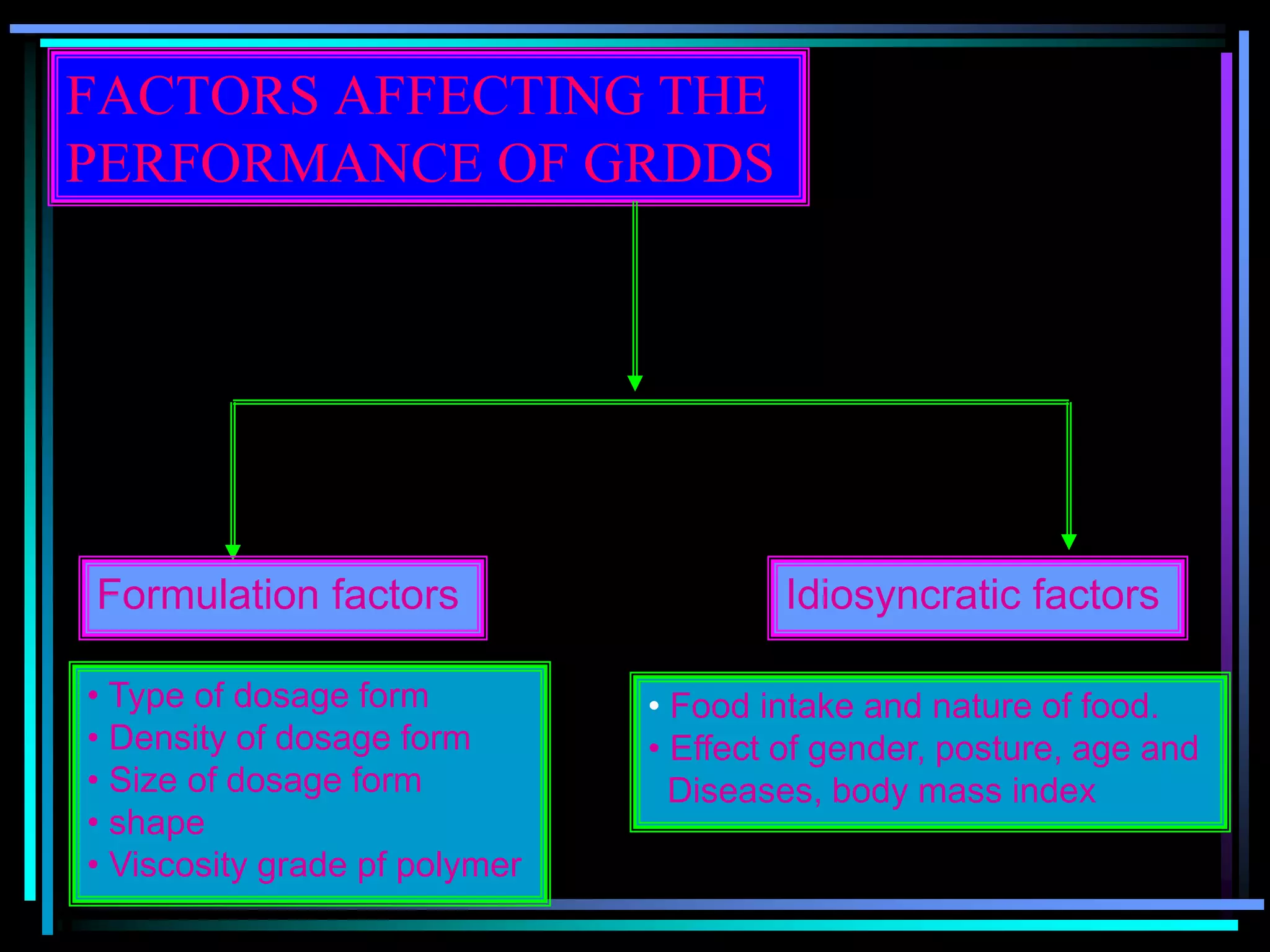
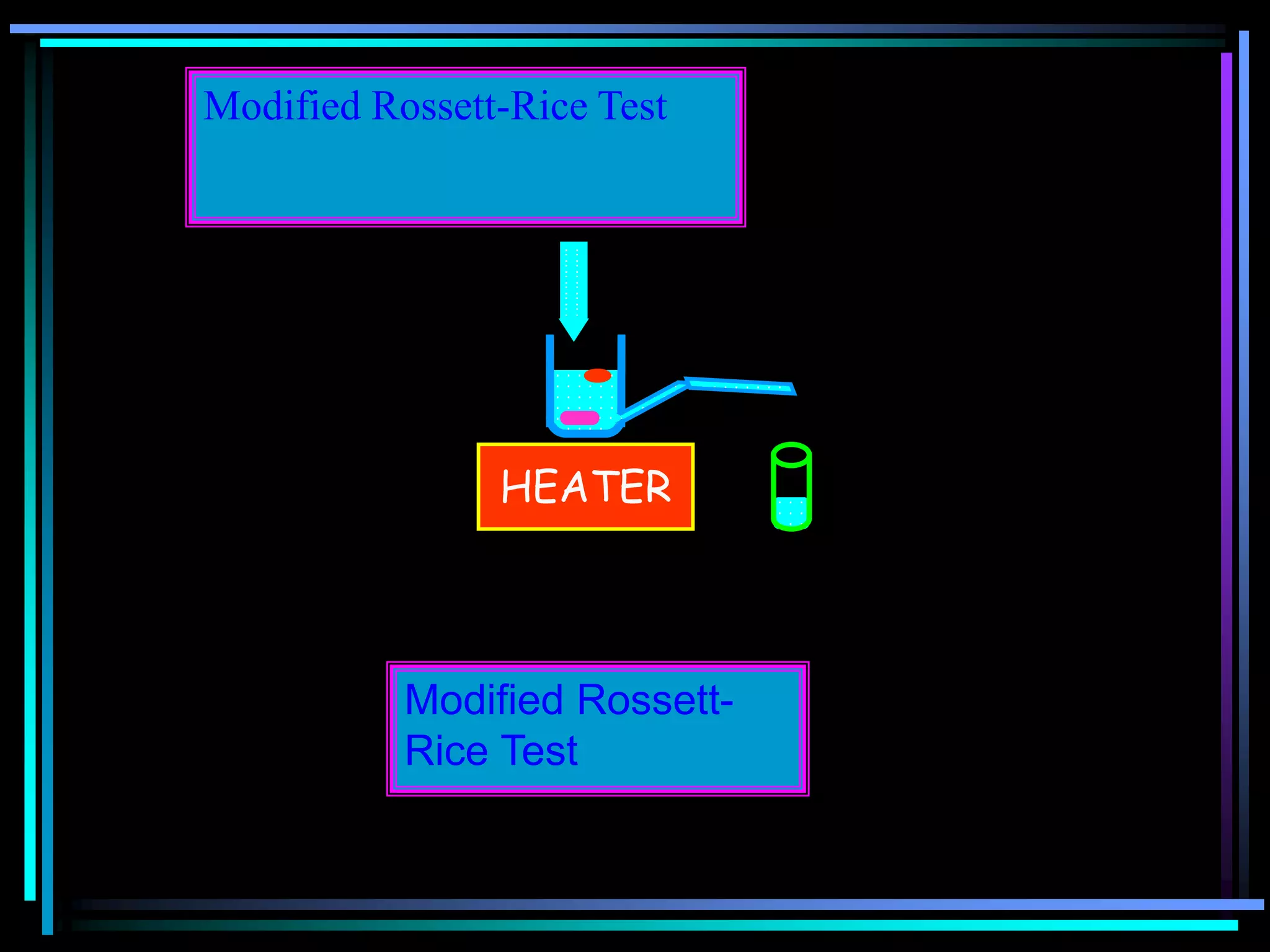
The document summarizes a seminar on gastroretentive drug delivery systems (GRDDS). It discusses the merits of GRDDS, including delivering drugs to the small intestine and improving bioavailability. Various gastroretentive technologies are described, including floating, expanding, bioadhesive, and high density systems. Factors affecting GRDDS performance and methods for evaluating different GRDDS are also outlined.



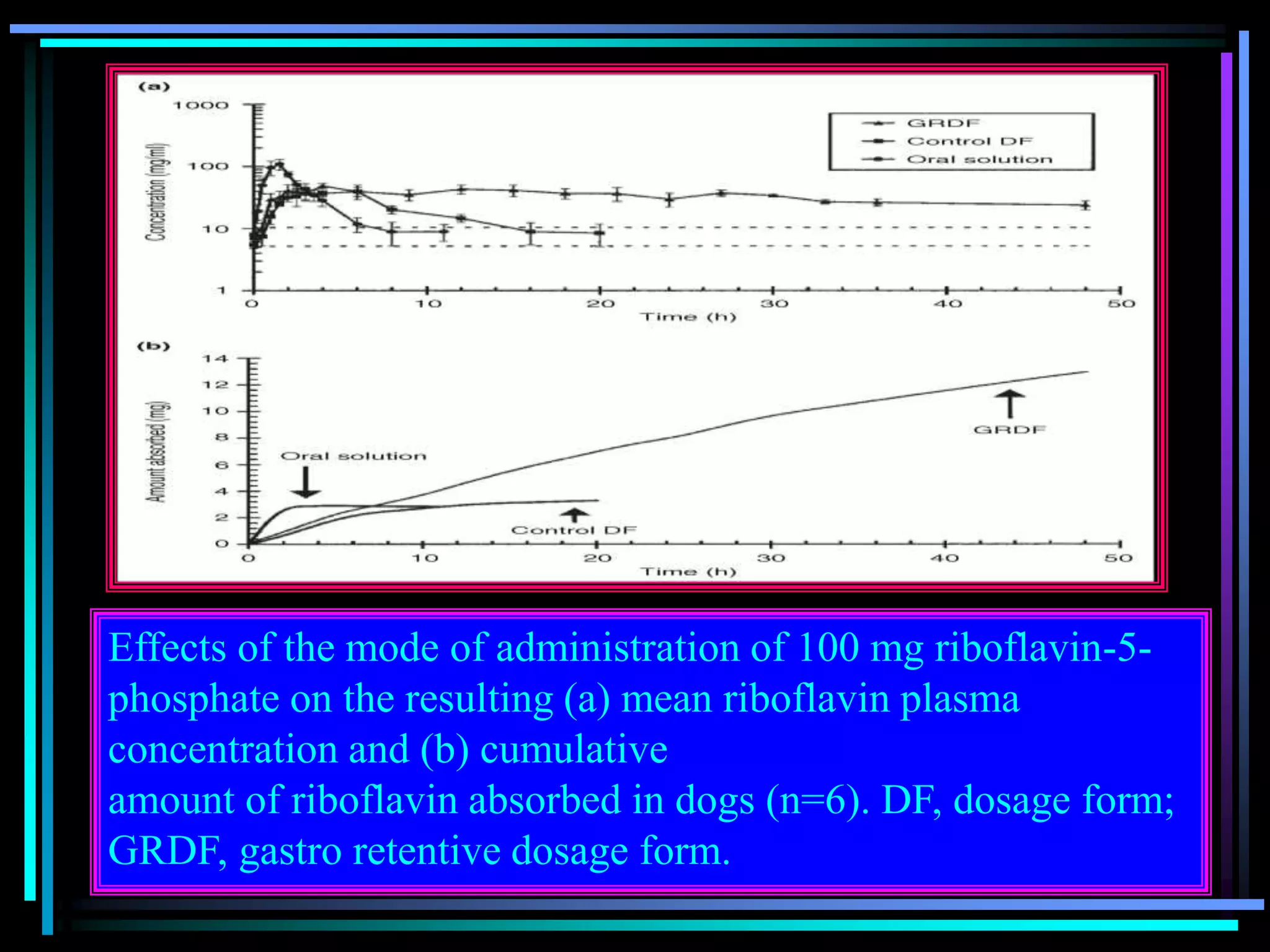

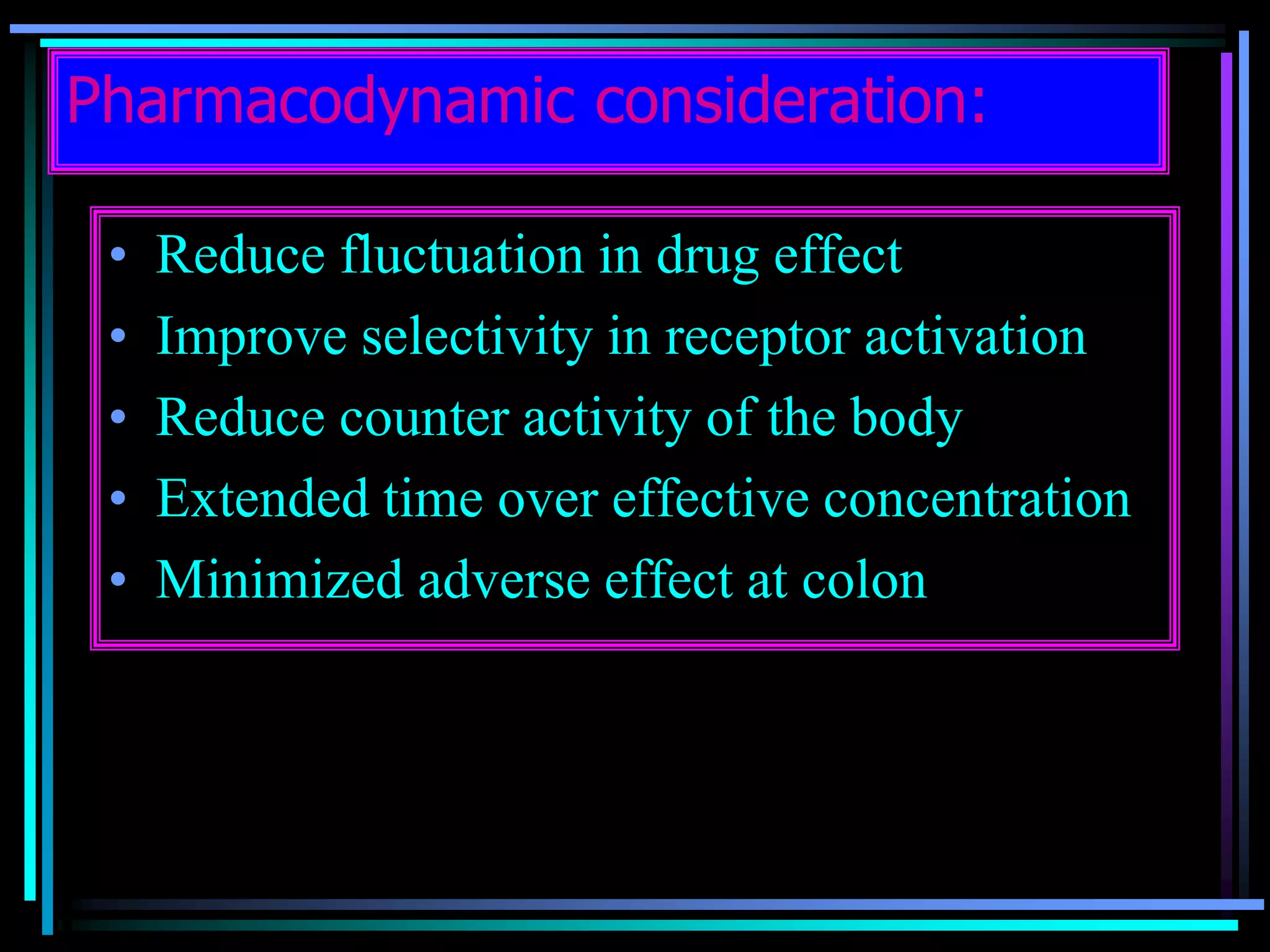

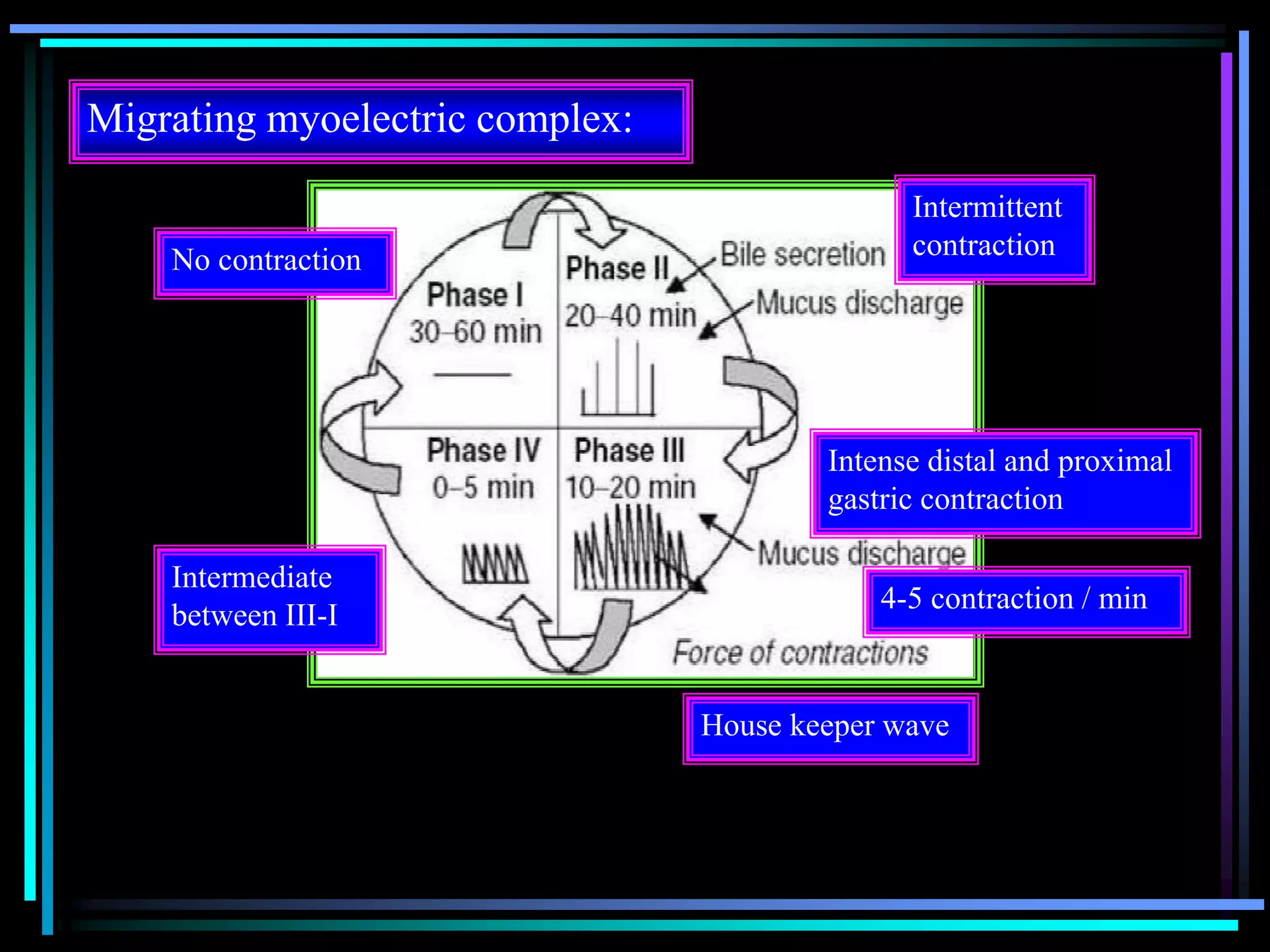


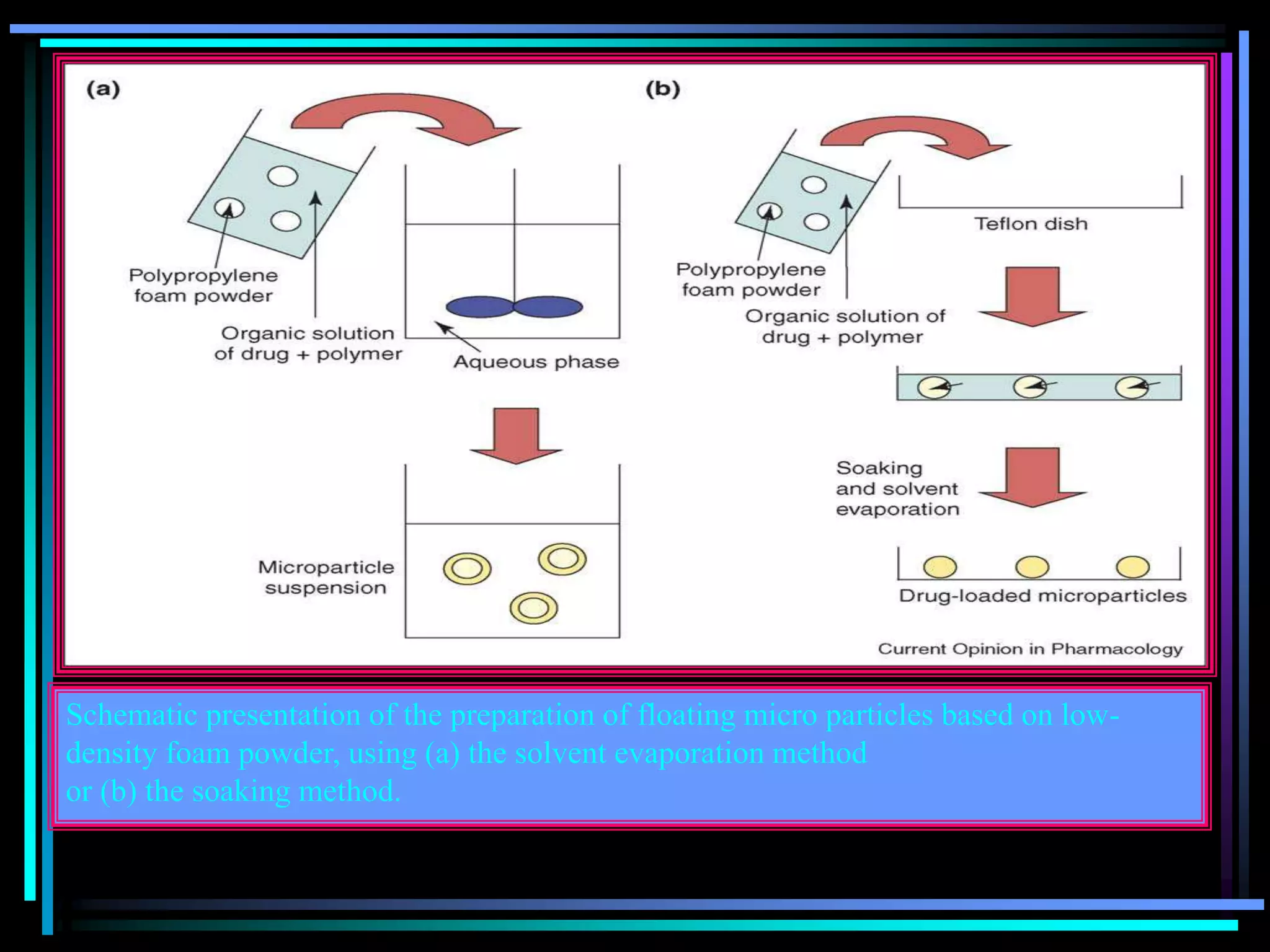
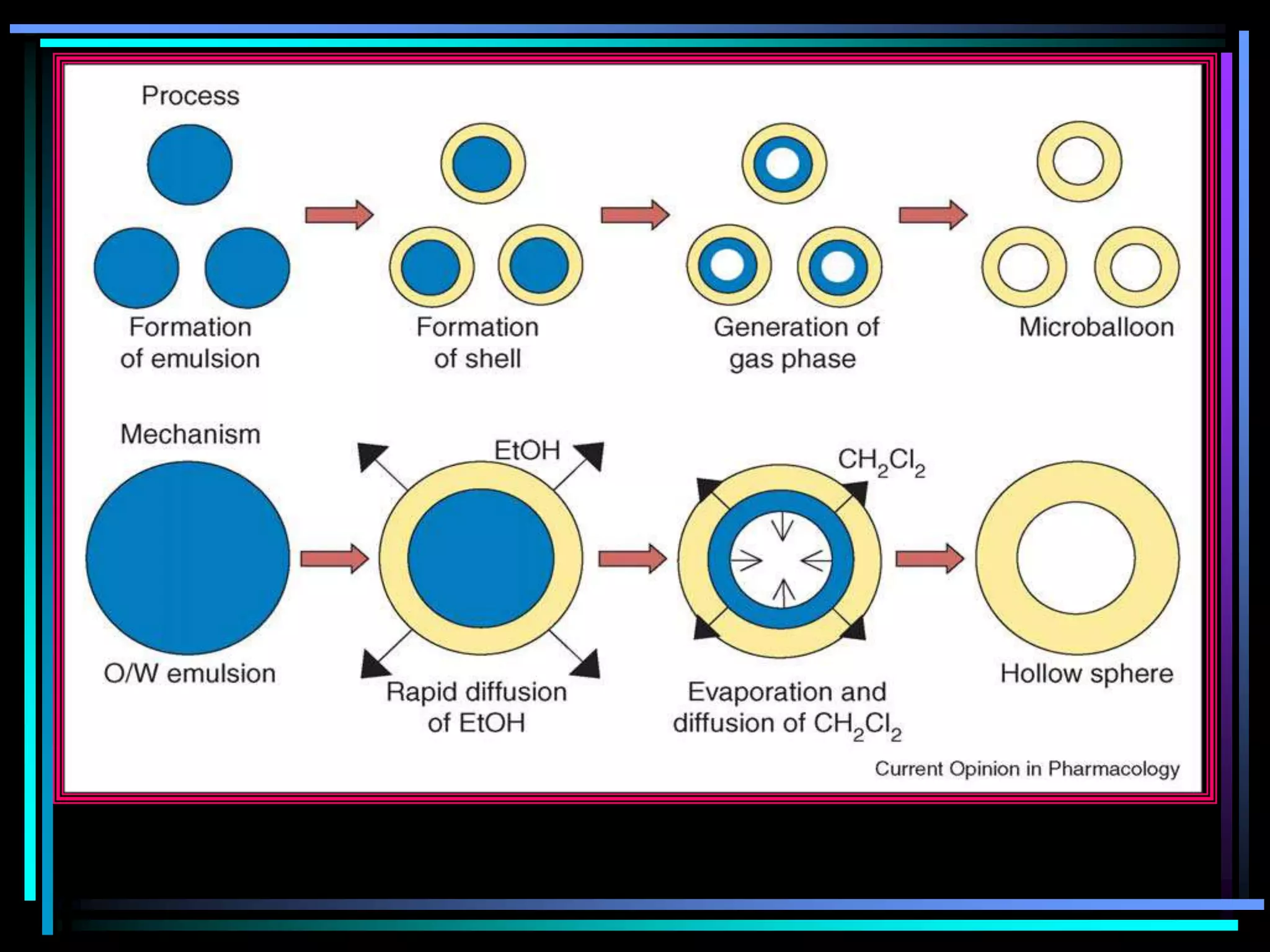
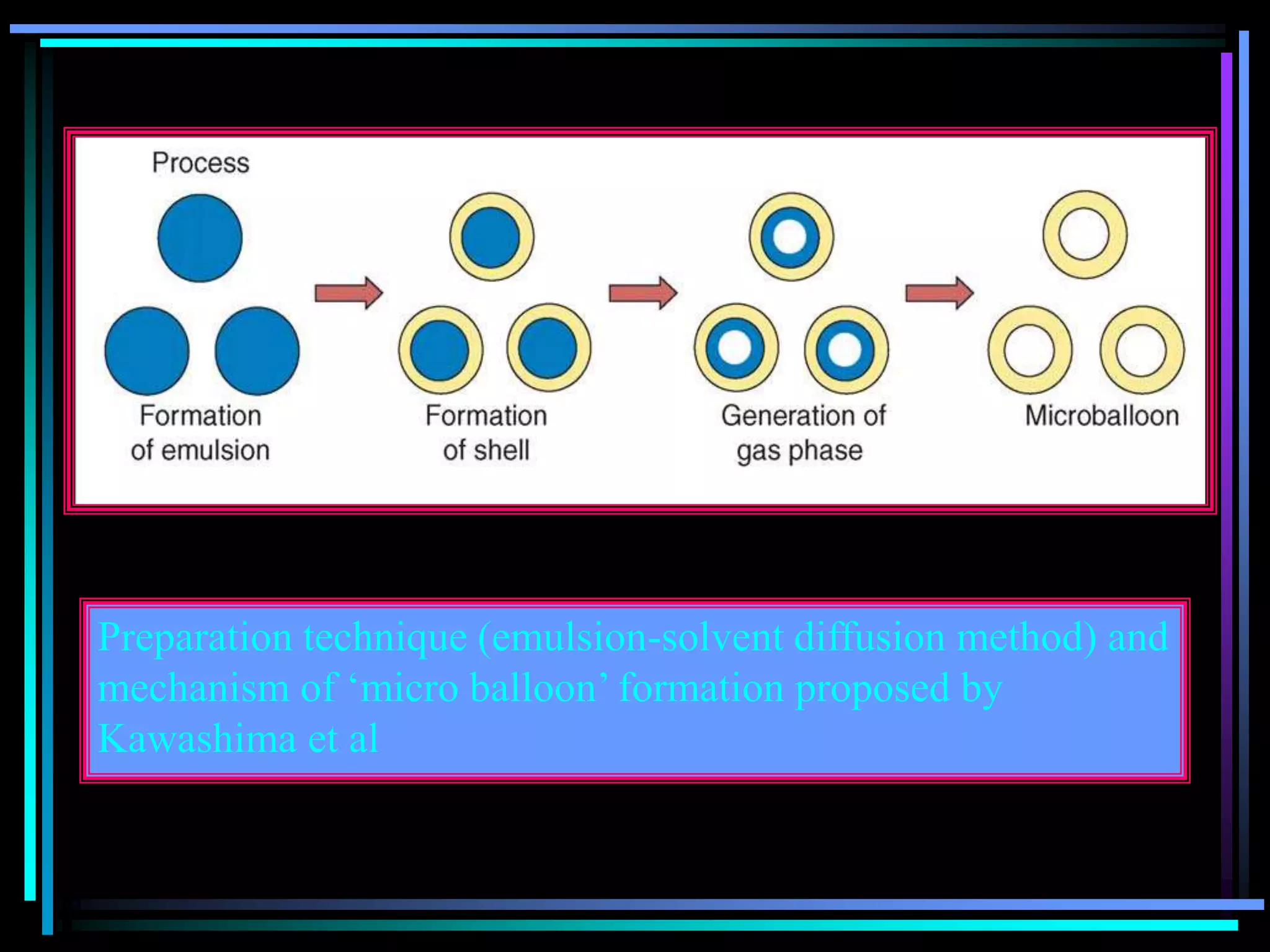
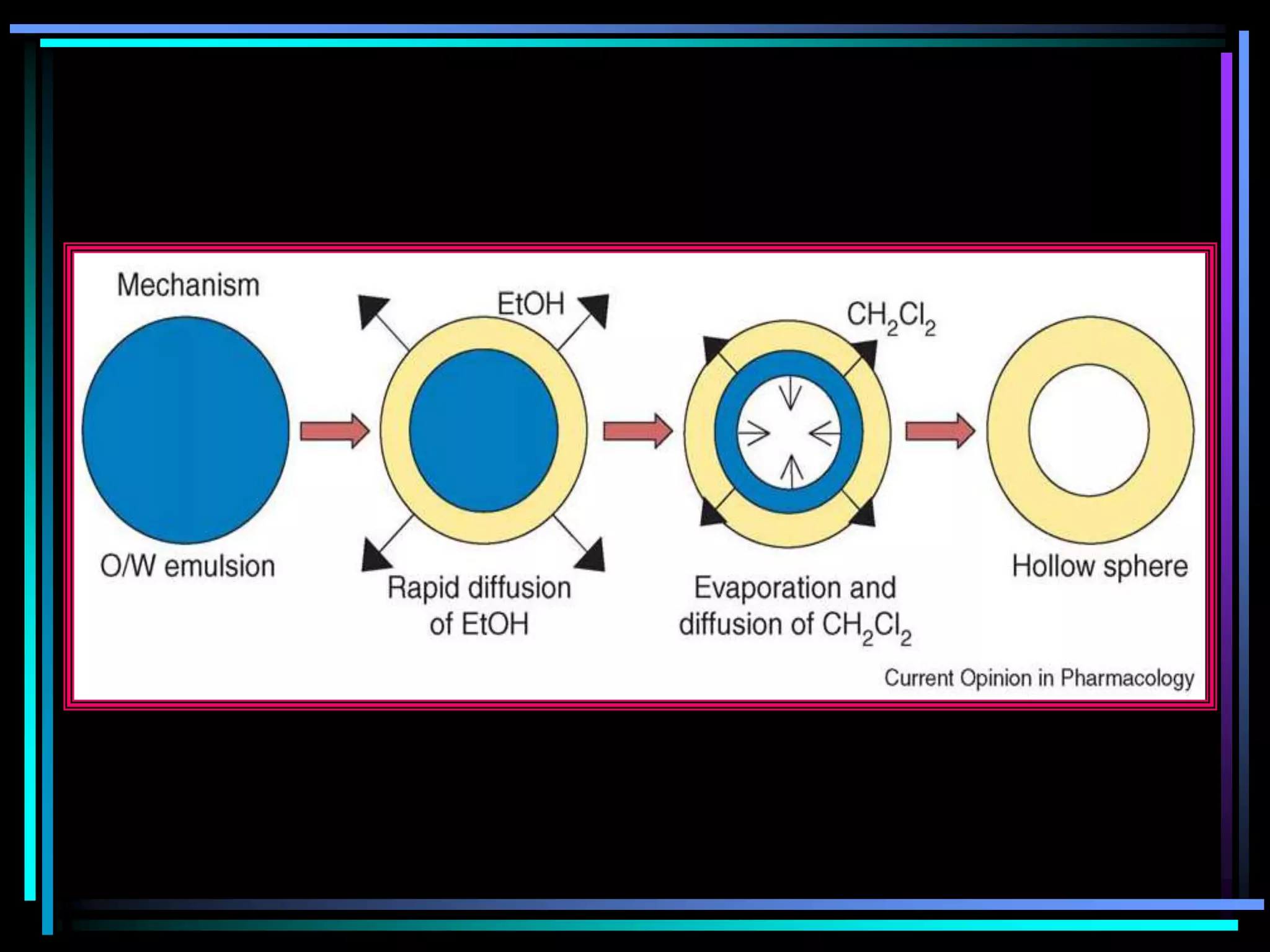
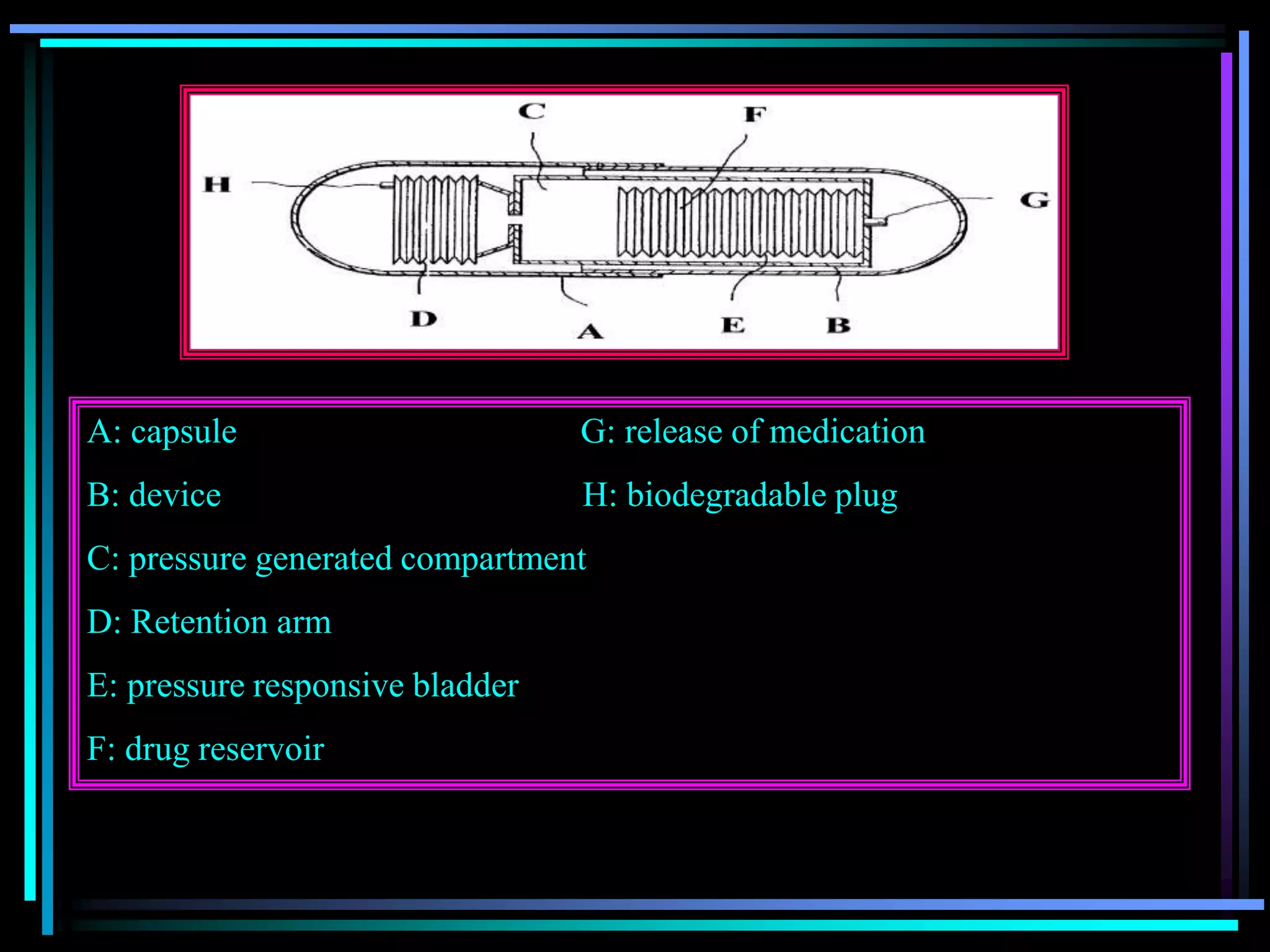
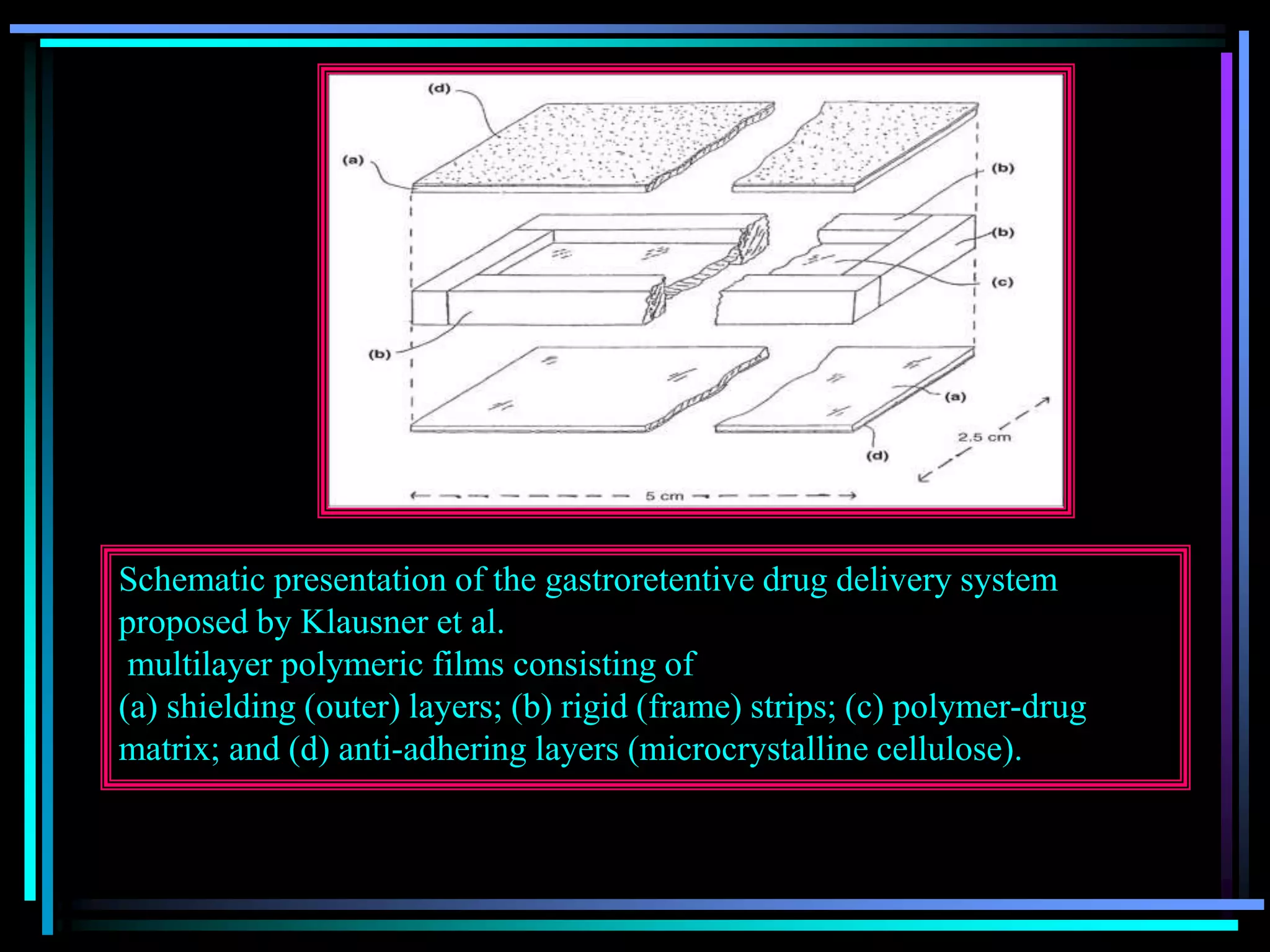
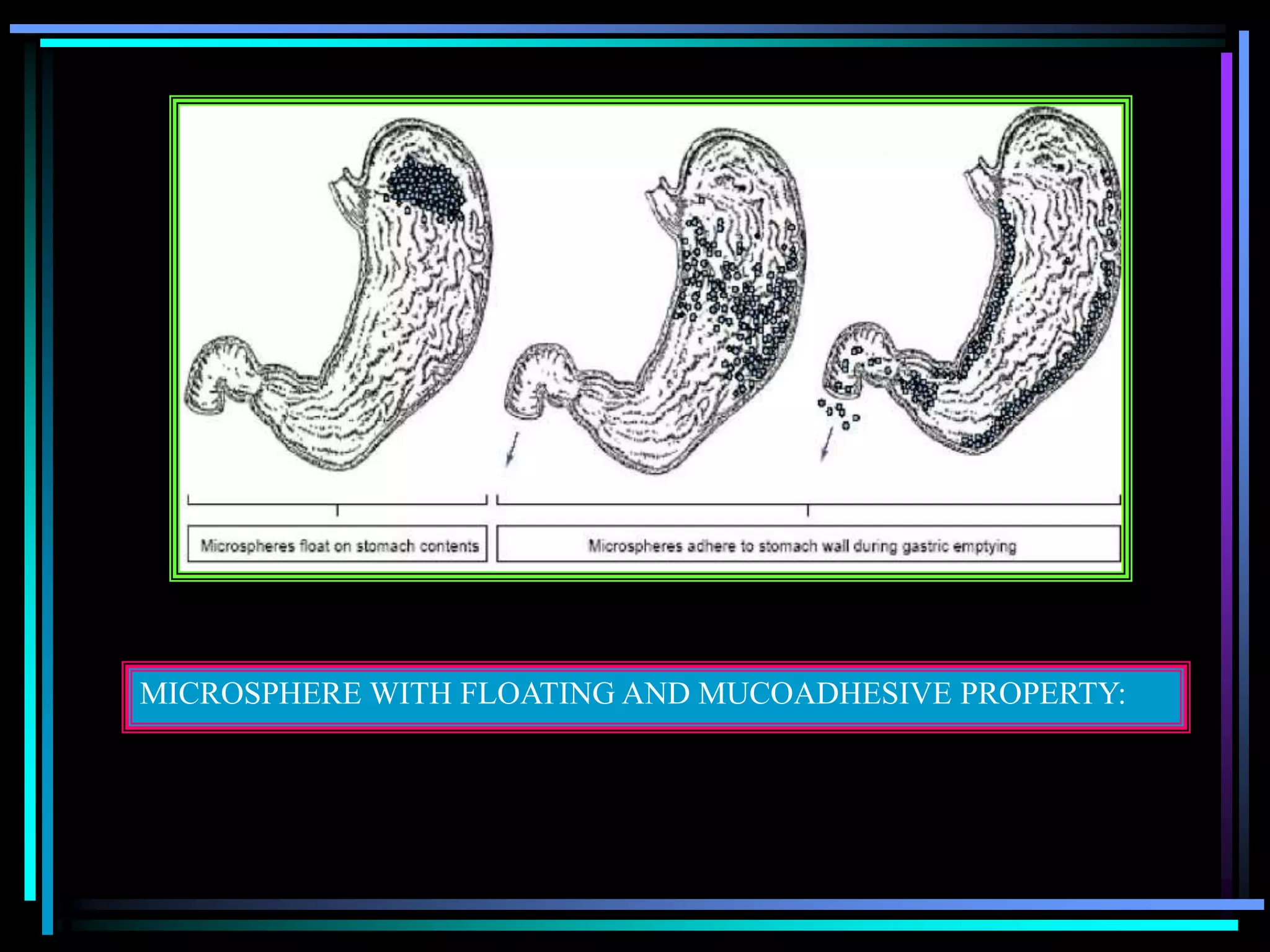





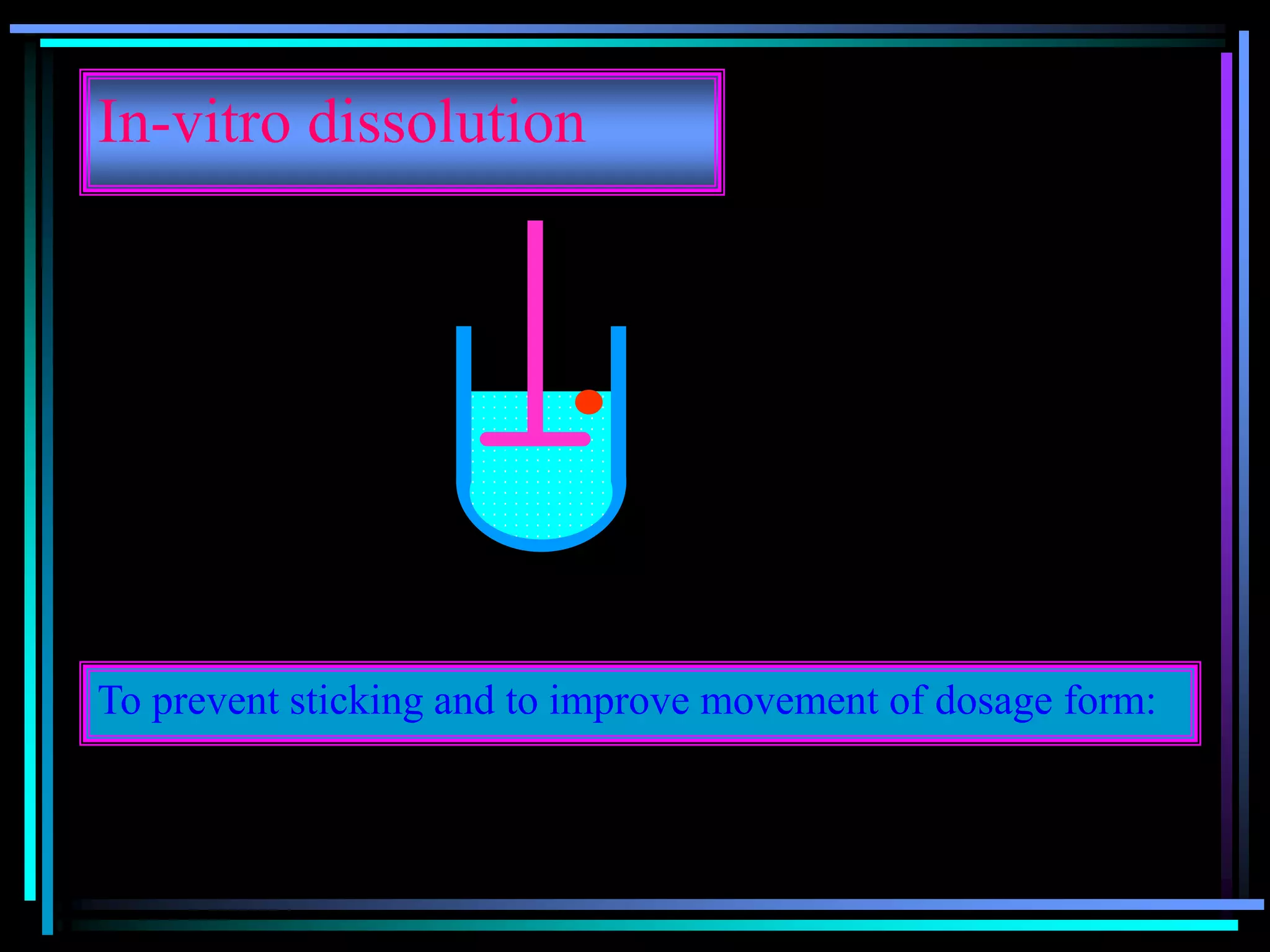
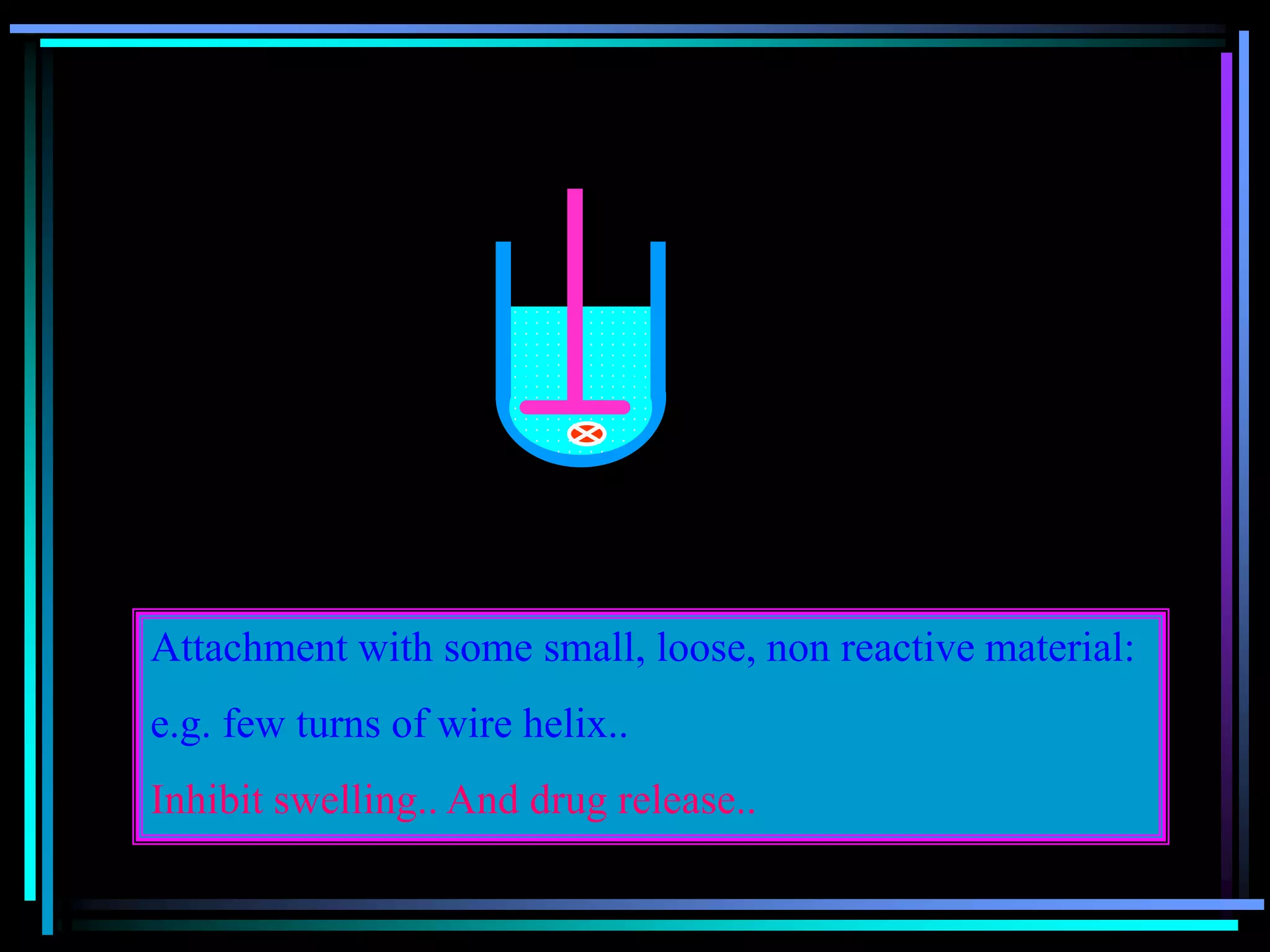
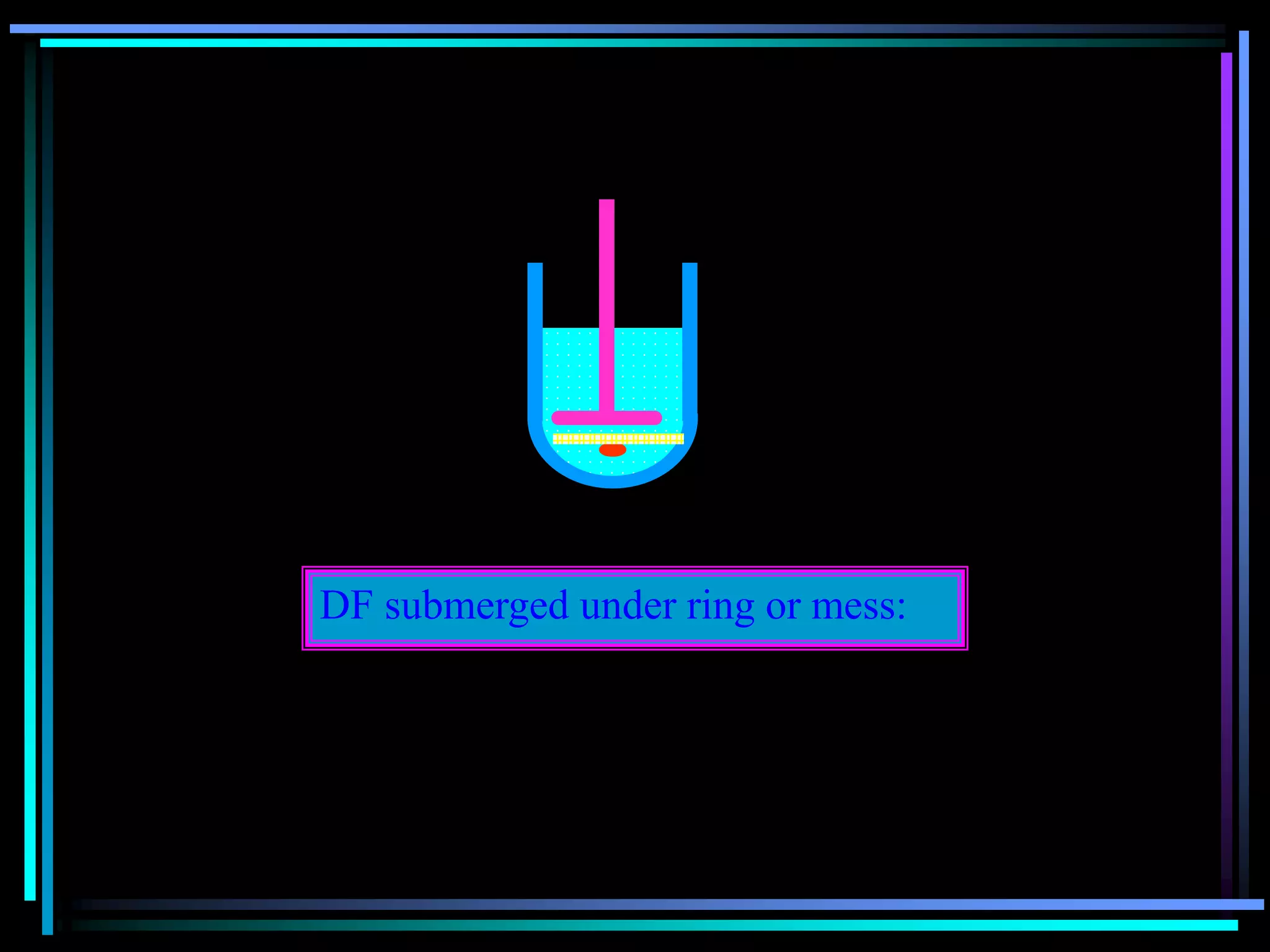
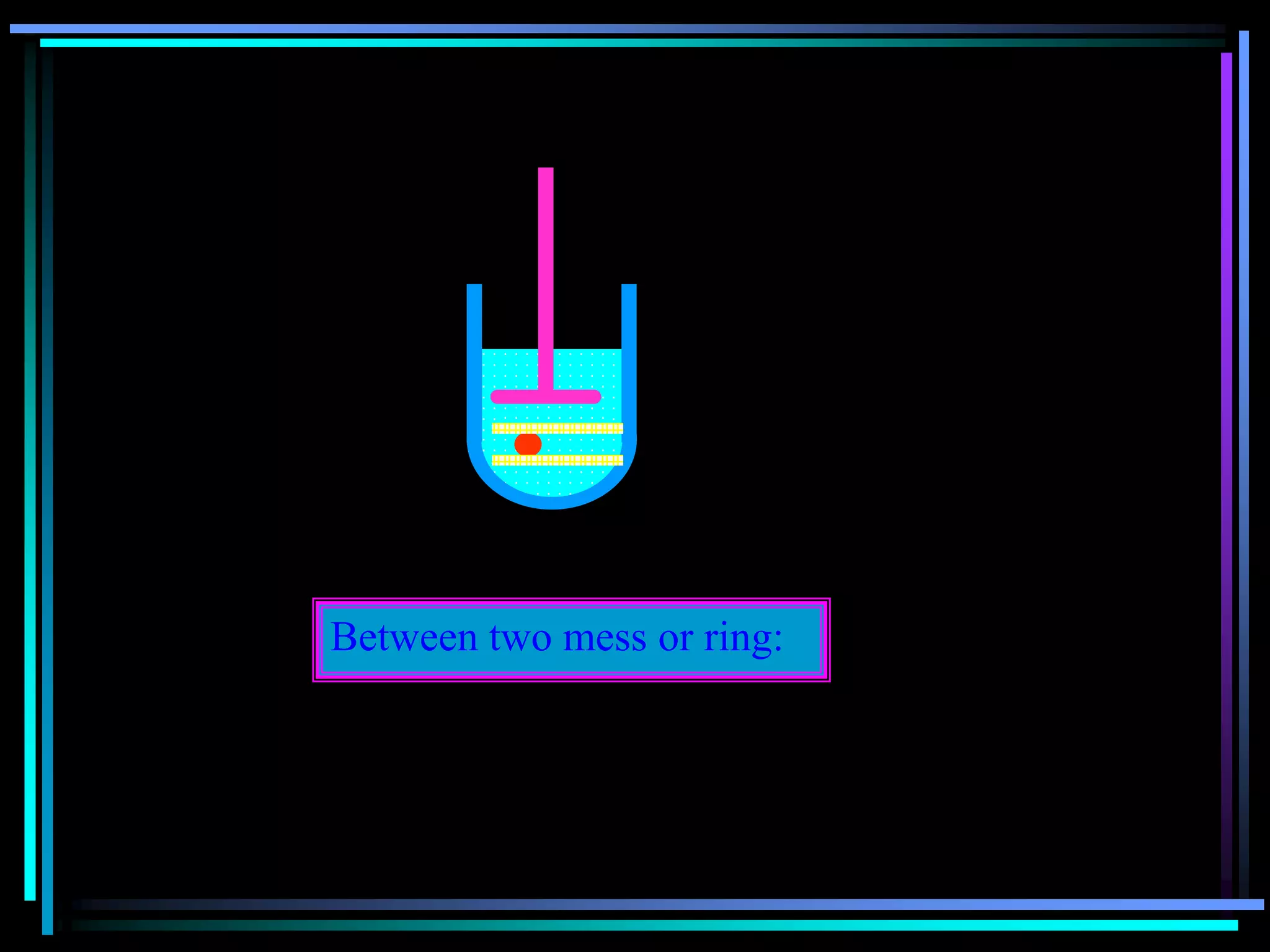

![Radiology:
• Its major advantages as compared to
-Scintigraphy are simplicity and cost. However, use of X-
ray experiment in biopharmaceutical studies involving
healthy volunteers [130]
• Barium sulphate high concentration:
e.g. 40%
• Aluminum thread contrast medium](https://image.slidesharecdn.com/grdds-200808165832/75/Chapter-on-Search-Results-Web-results-Gastro-retentive-drug-delivery-system-GRDDS-71-2048.jpg)