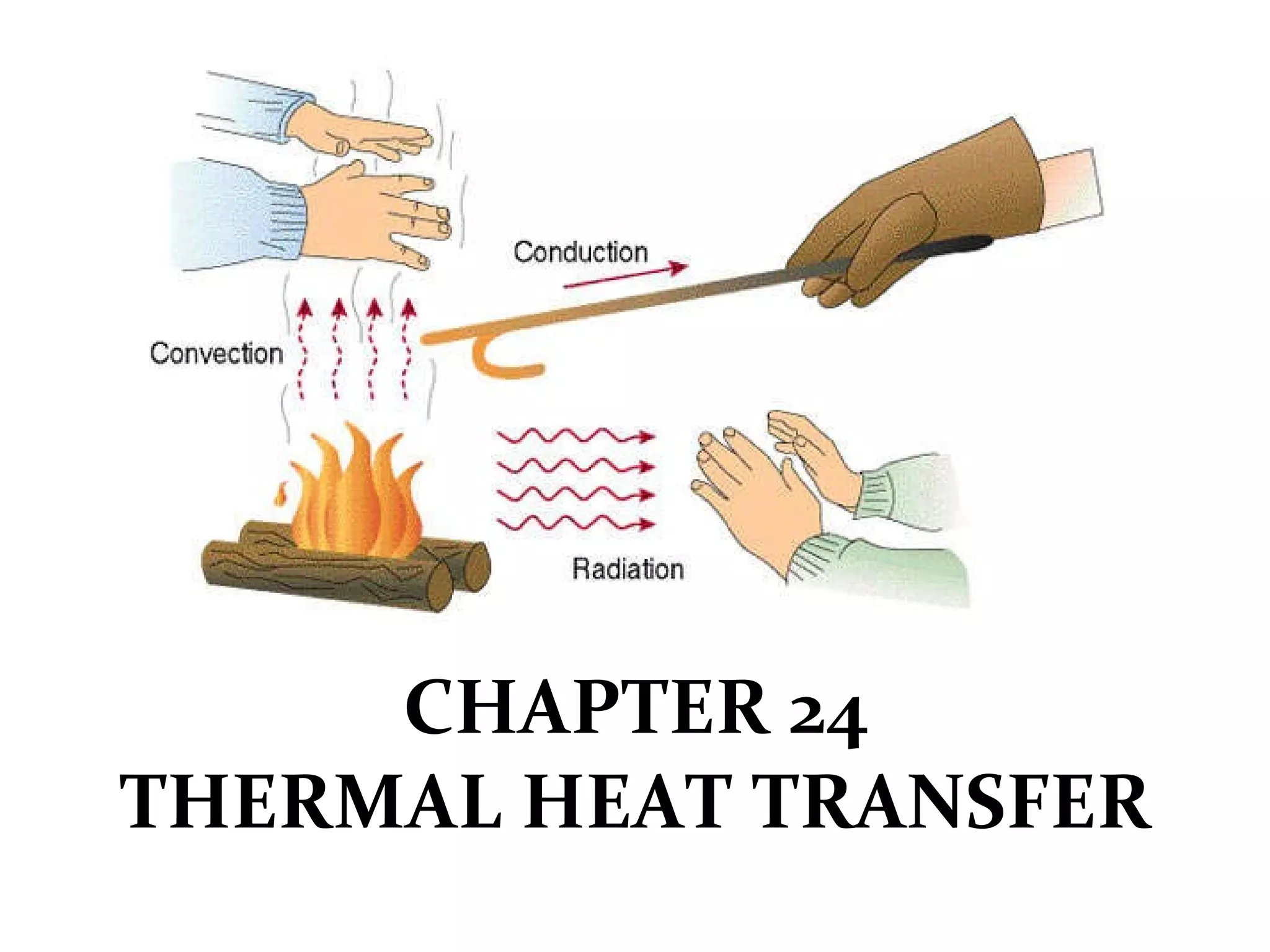
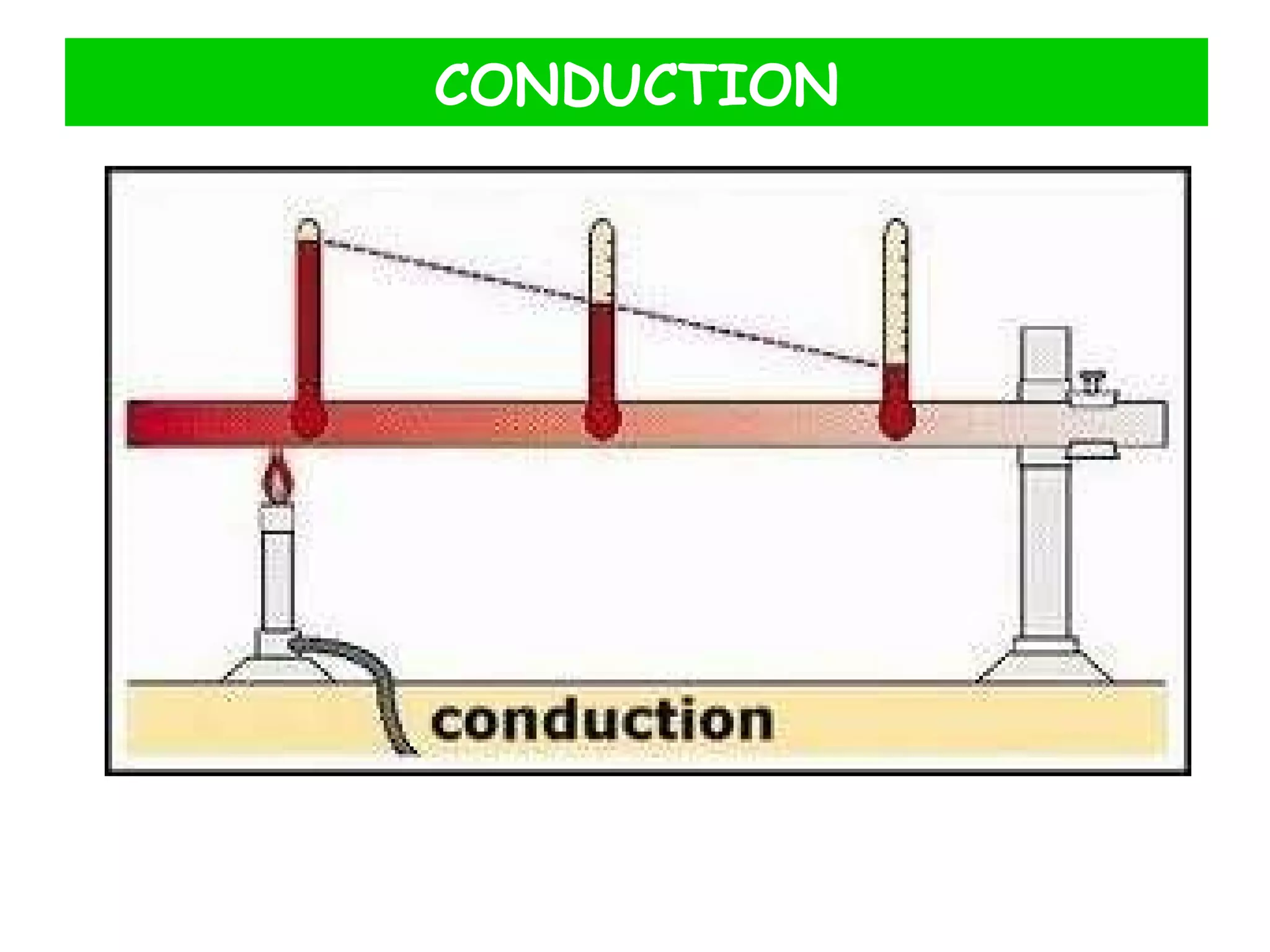
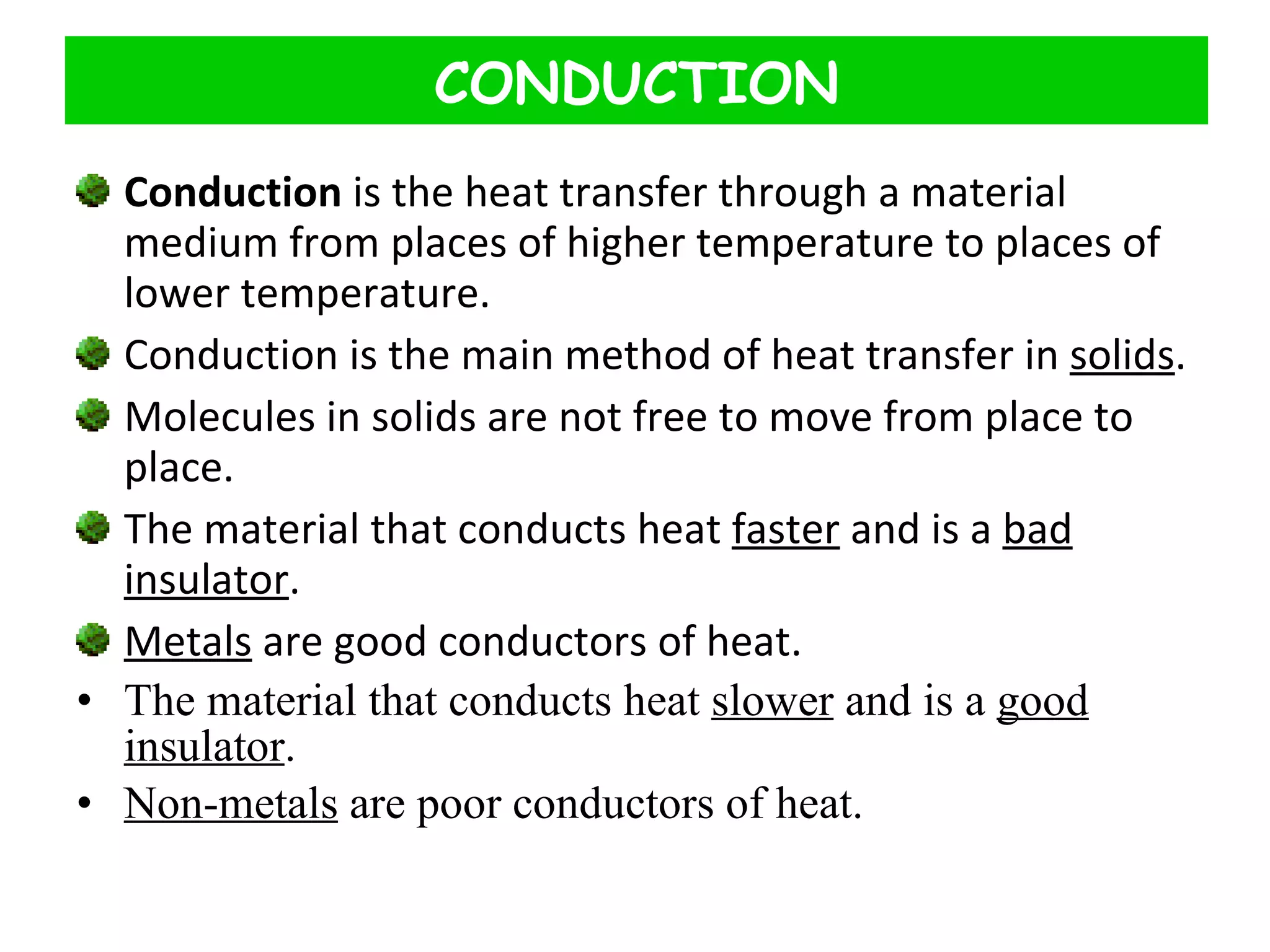
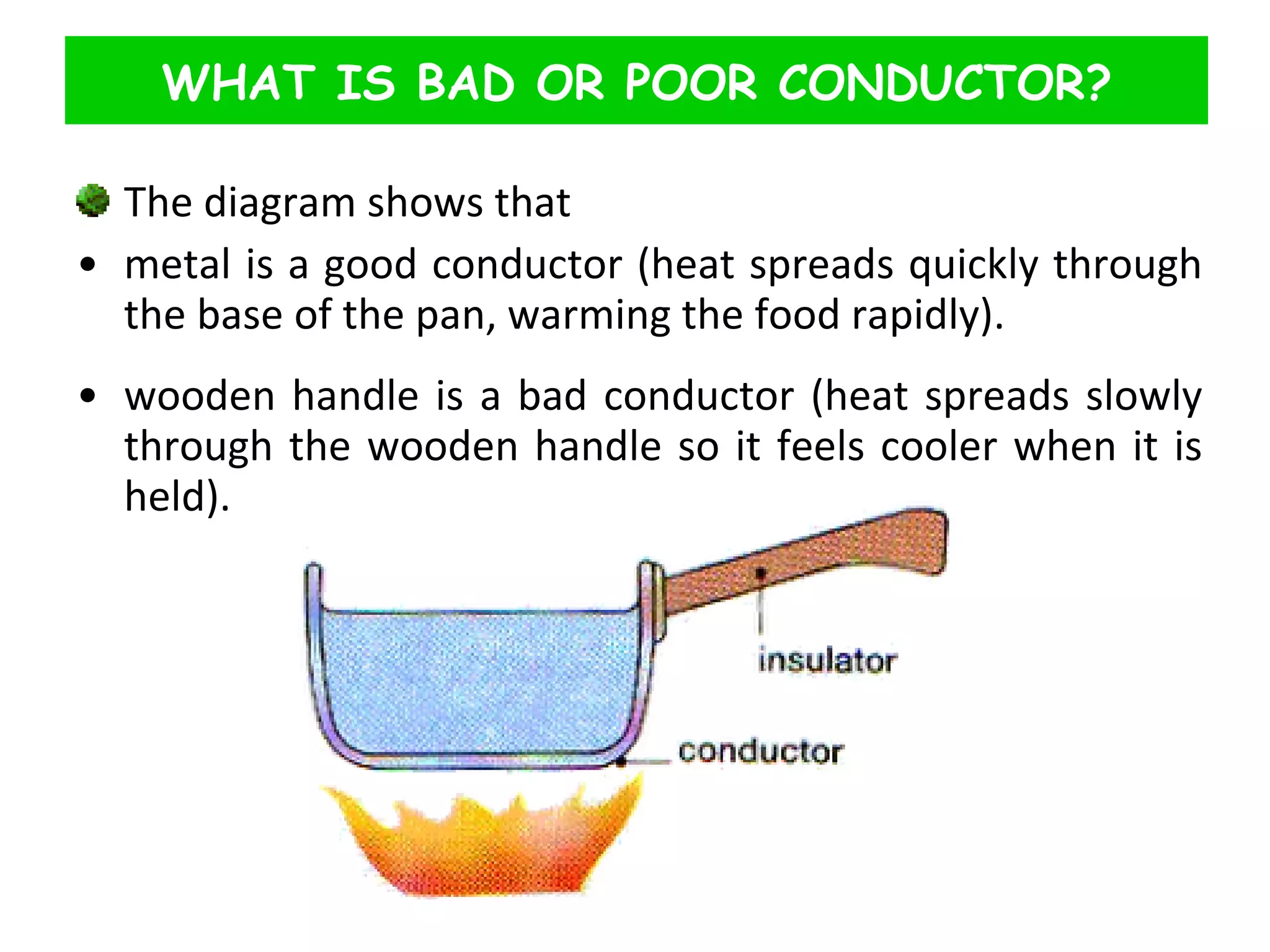
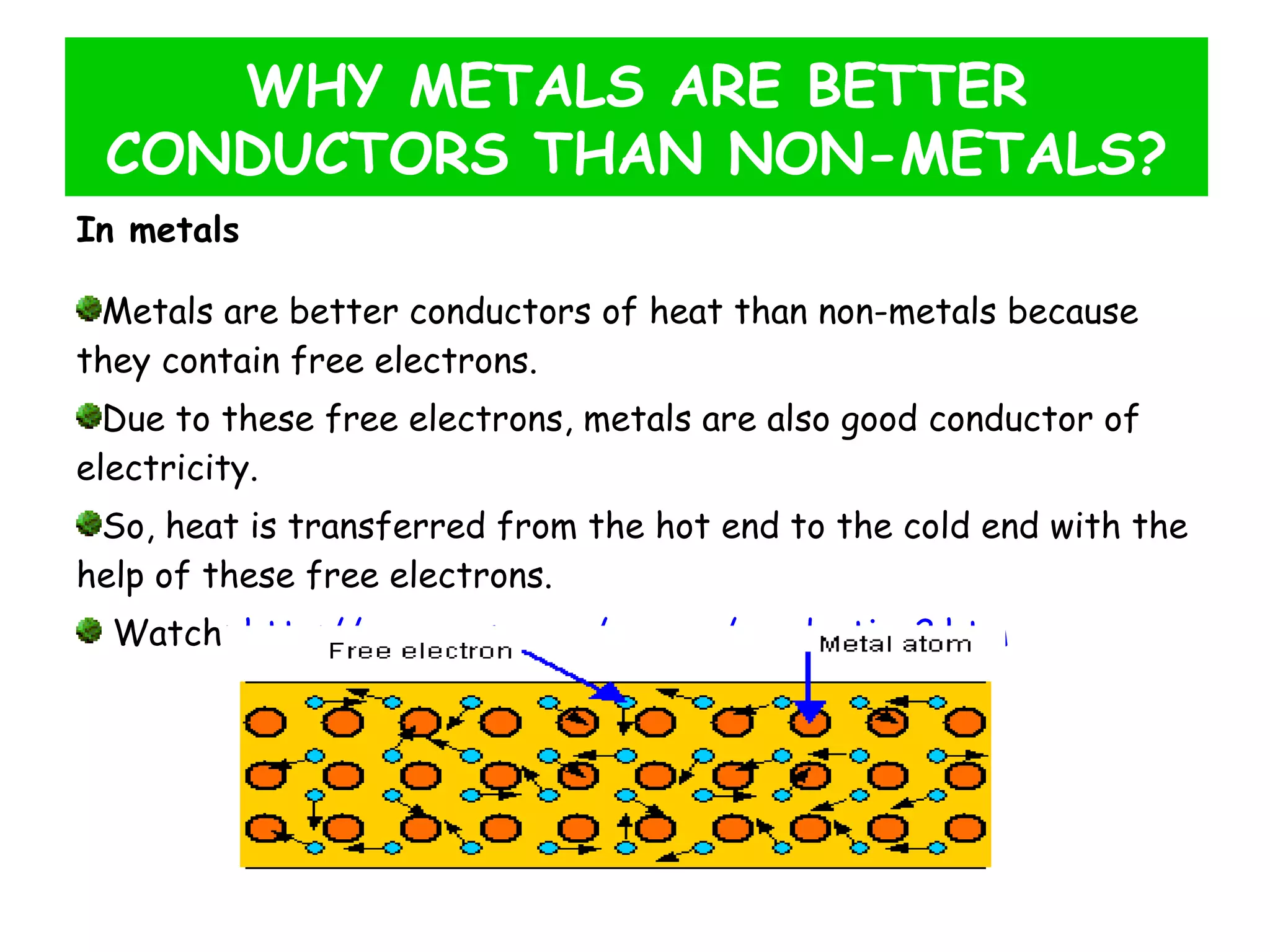
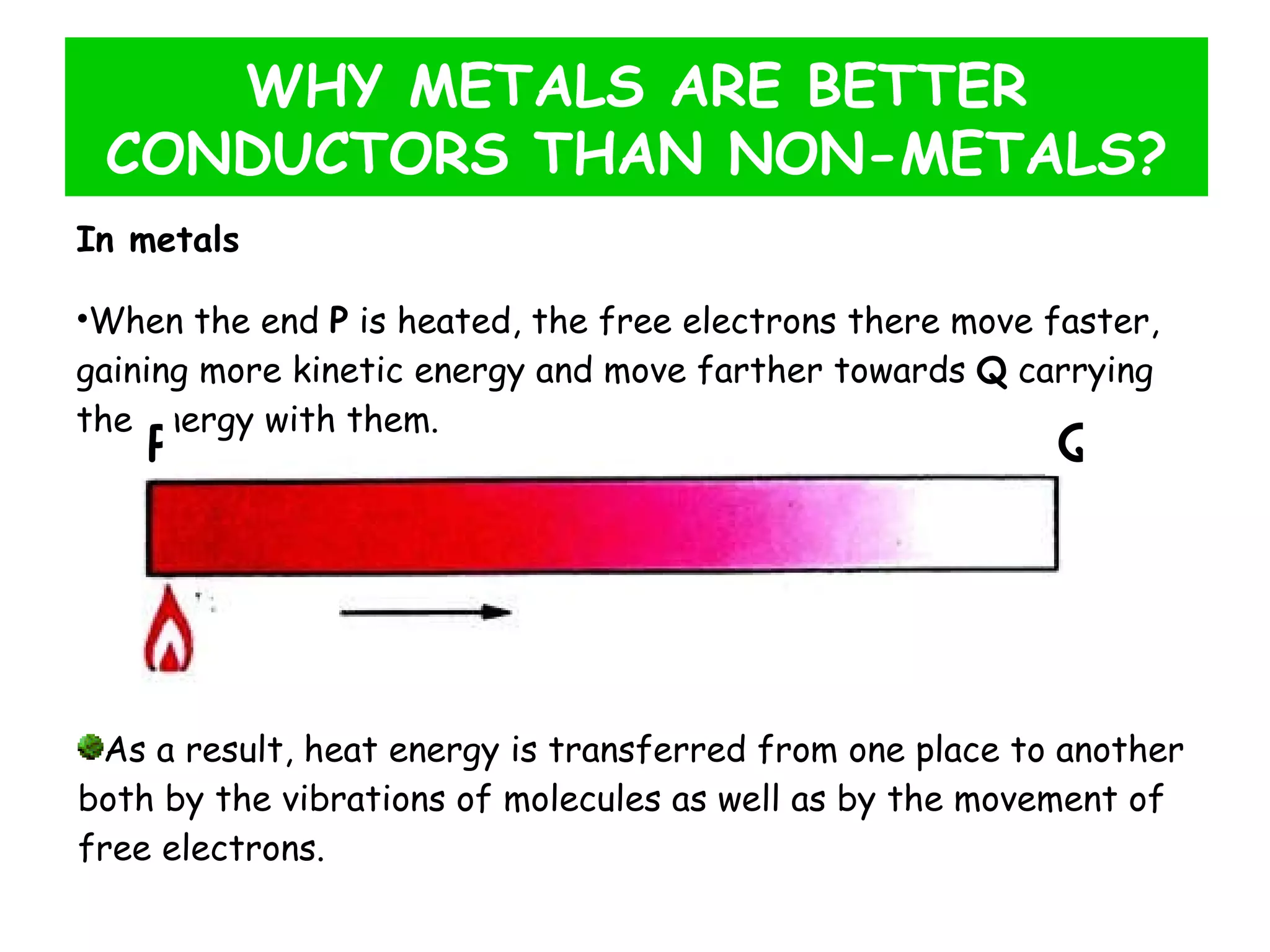
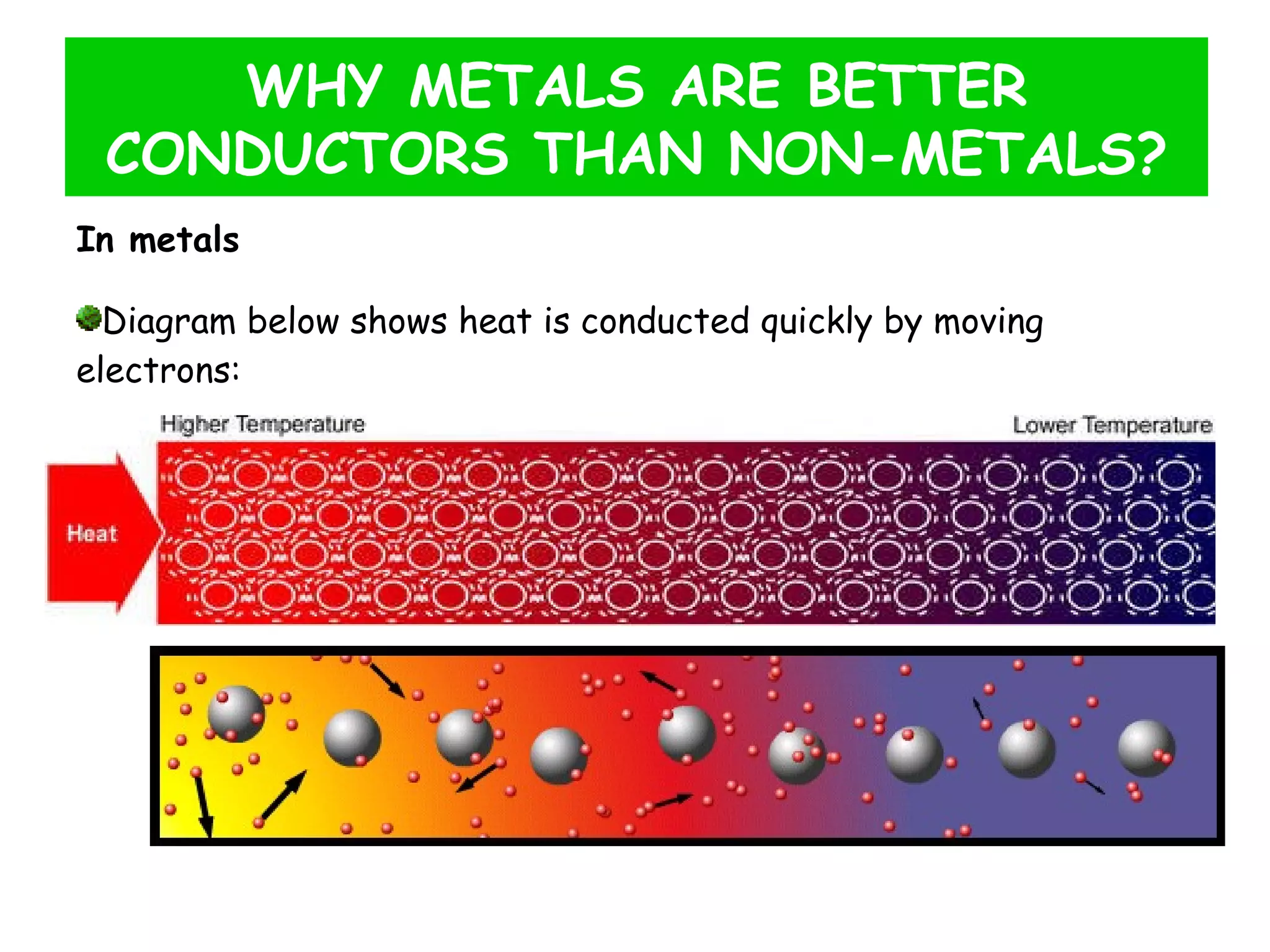
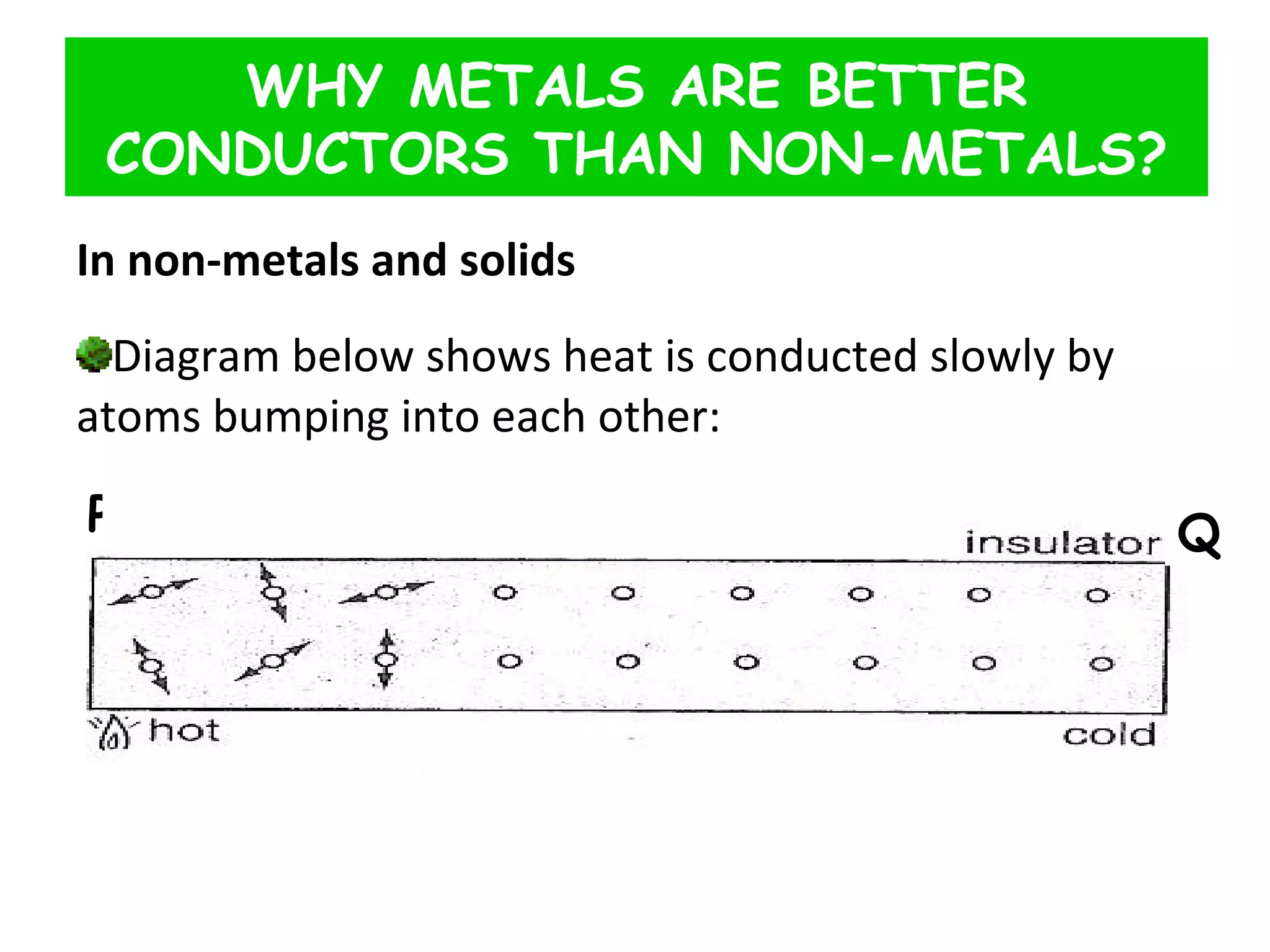
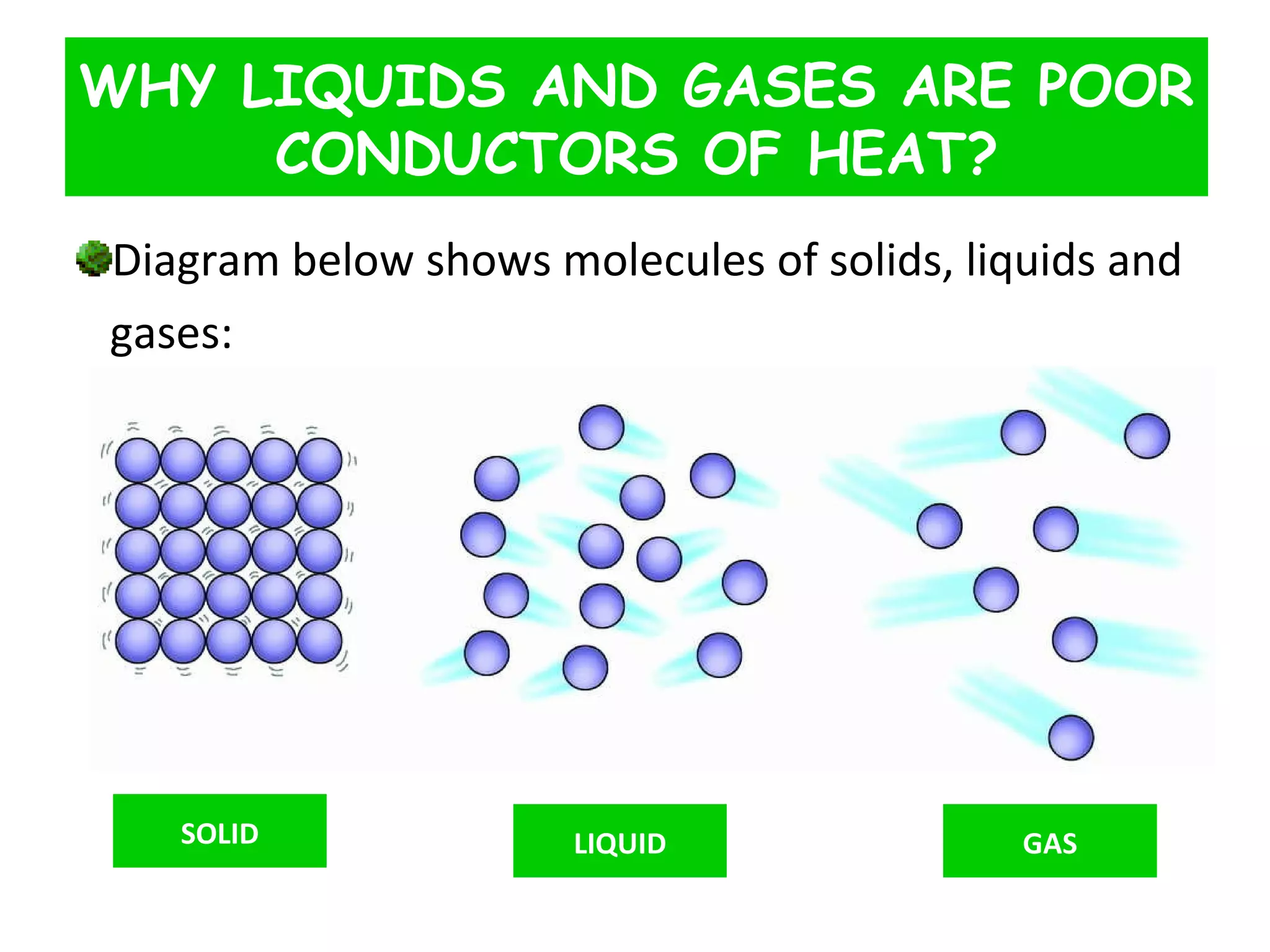
This document discusses heat transfer through conduction. It explains that conduction is the transfer of heat through a material from higher to lower temperature areas without movement of the material. Metals are good conductors of heat due to their free electrons, while non-metals and insulators conduct heat more slowly through molecular vibration. Liquids and gases are poor conductors because their molecules are more spaced out than in solids.