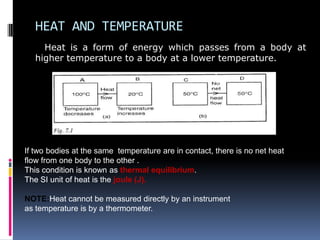
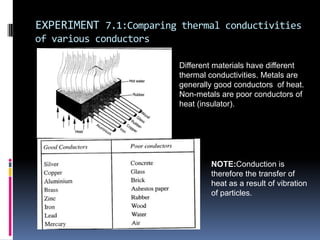
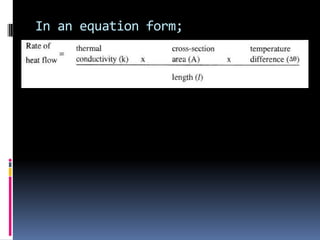
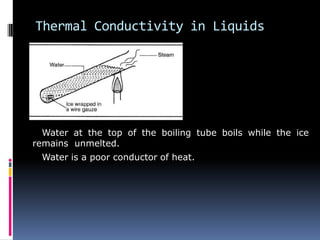
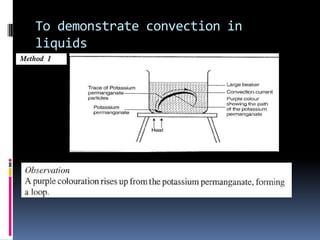
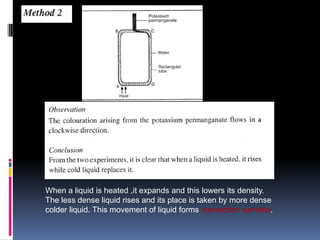
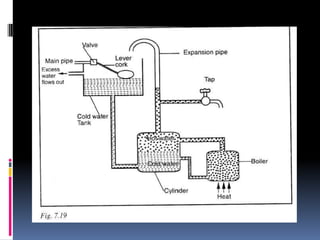
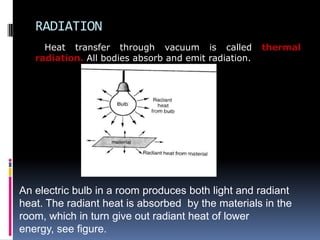
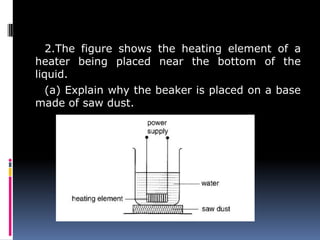
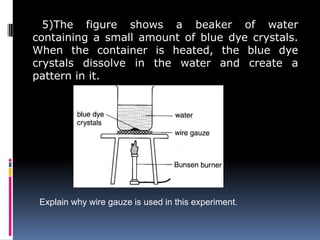
Heat can be transferred through three modes: conduction, convection, and radiation. Conduction requires physical contact between objects and involves heat transfer through vibrations in solids. Convection involves the movement of heated fluids like liquids and gases. Radiation transfers heat through electromagnetic waves and does not require a medium. Different materials conduct heat at different rates depending on properties like their molecular structure. Metals are generally good conductors while gases are poor conductors due to low molecular cohesion. Proper use of these conductive properties allows for efficient heat transfer in applications like cooking.