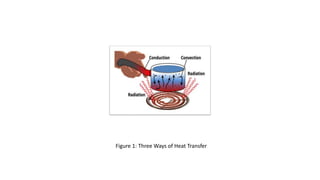
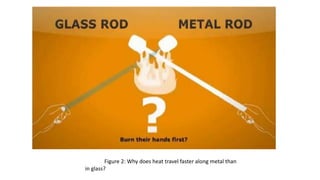
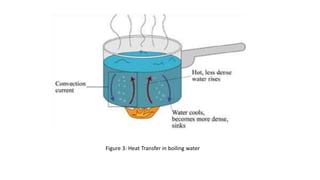
This document discusses different methods of heat transfer, specifically conduction and convection. It defines conduction as the direct transfer of heat between objects in contact, explaining that it occurs faster in metals due to their structure and free-moving electrons. Convection is defined as the transfer of heat by the movement of fluids like liquids and gases, such as in boiling water where hot water rises and cold sinks, creating convection currents. The document provides illustrations of these processes and their practical applications.