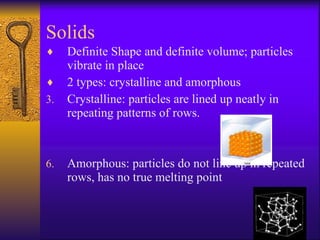
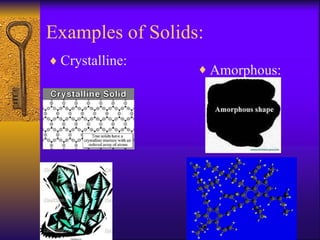
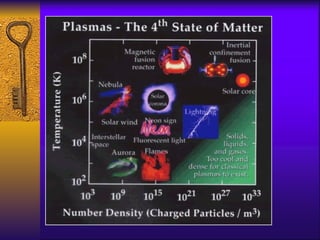
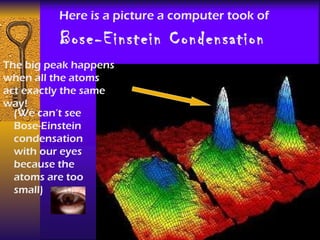
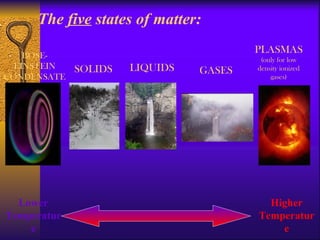
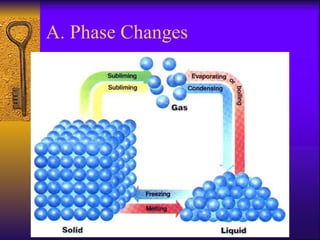
1. The document describes the five states of matter - solids, liquids, gases, plasmas, and Bose-Einstein condensates. It explains the properties of each state and the phase changes between states.
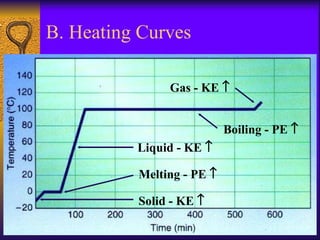
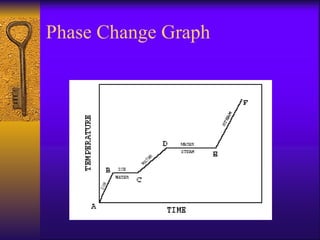
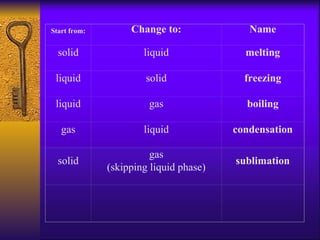
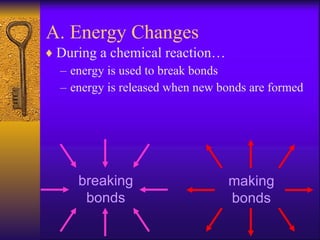
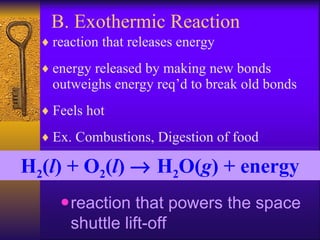
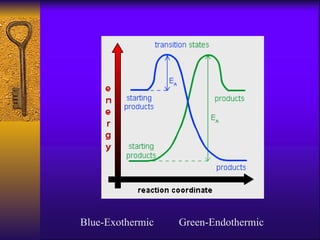
2. Heating curves are discussed, showing how energy absorption or release occurs during phase changes like melting and boiling. The concepts of endothermic and exothermic reactions in relation to energy changes are introduced.
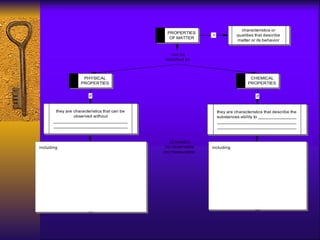
3. Chemical and physical properties and changes are defined, including examples like density, reactivity, physical changes like dissolving, and chemical changes like burning or rusting. Characteristics used to identify substances are outlined.