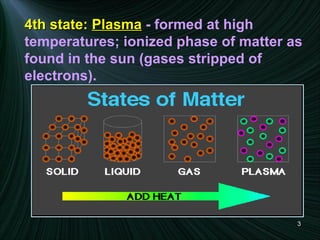
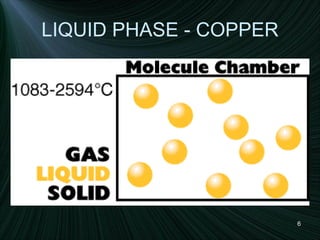
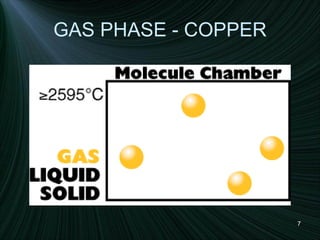
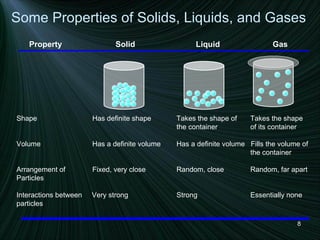
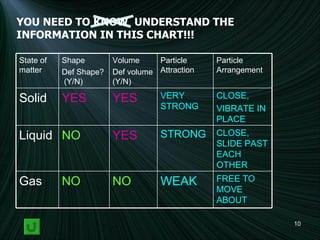
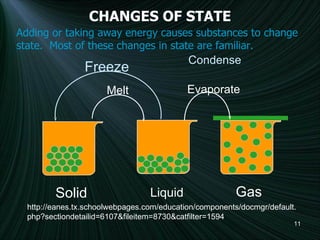
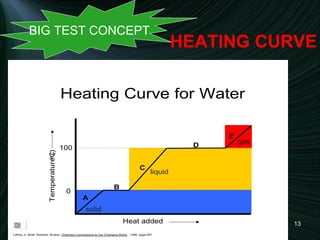
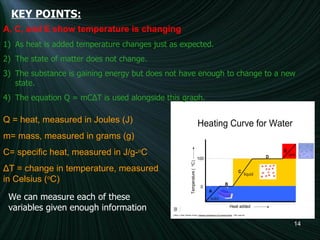
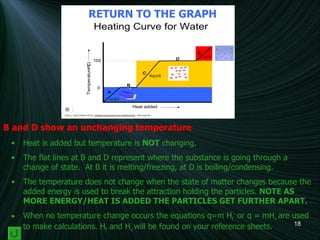
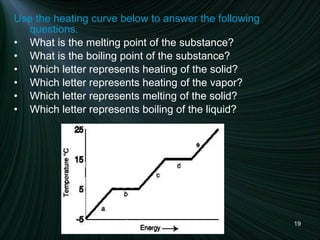
The document discusses the different states of matter and changes between states. It includes:
1) Descriptions of solids, liquids, and gases in terms of particle arrangement, volume, shape, and interactions.
2) Explanations of changes in state like melting, freezing, evaporation, and condensation that occur when energy is added or removed from a substance.
3) A diagram of a heating curve showing how temperature changes as energy is added for different substances and states. Sections with no temperature change represent changes in state.