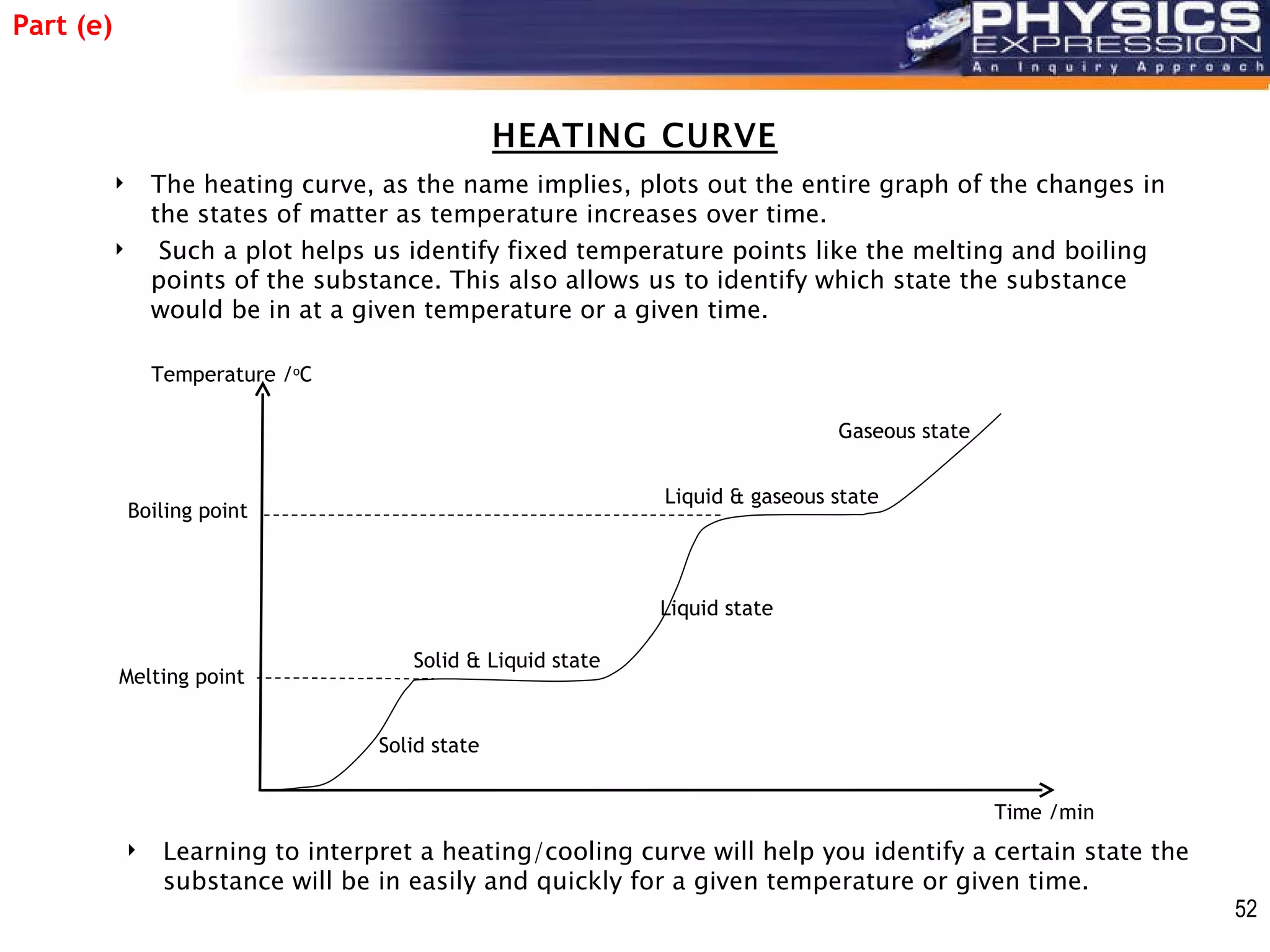
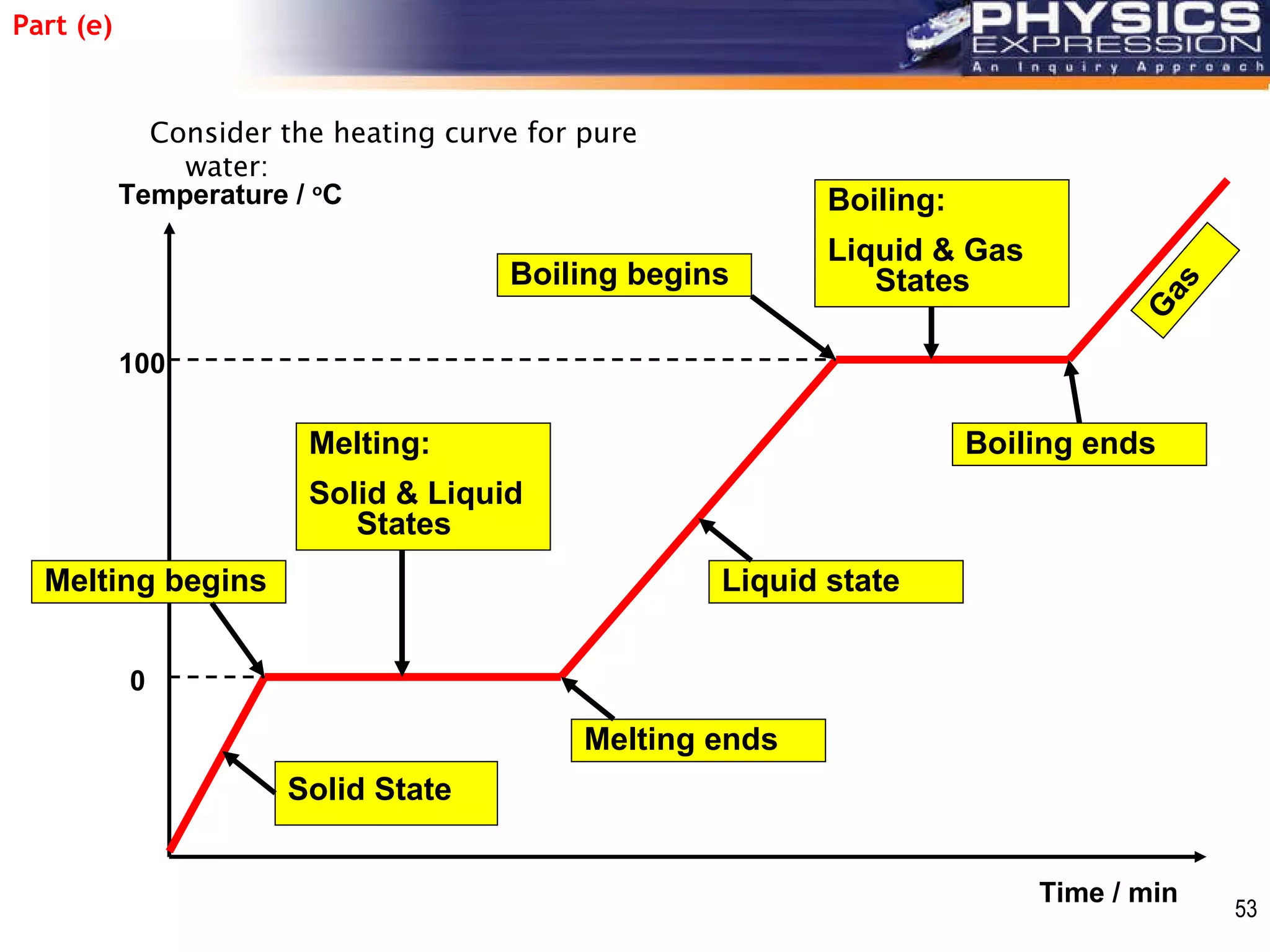
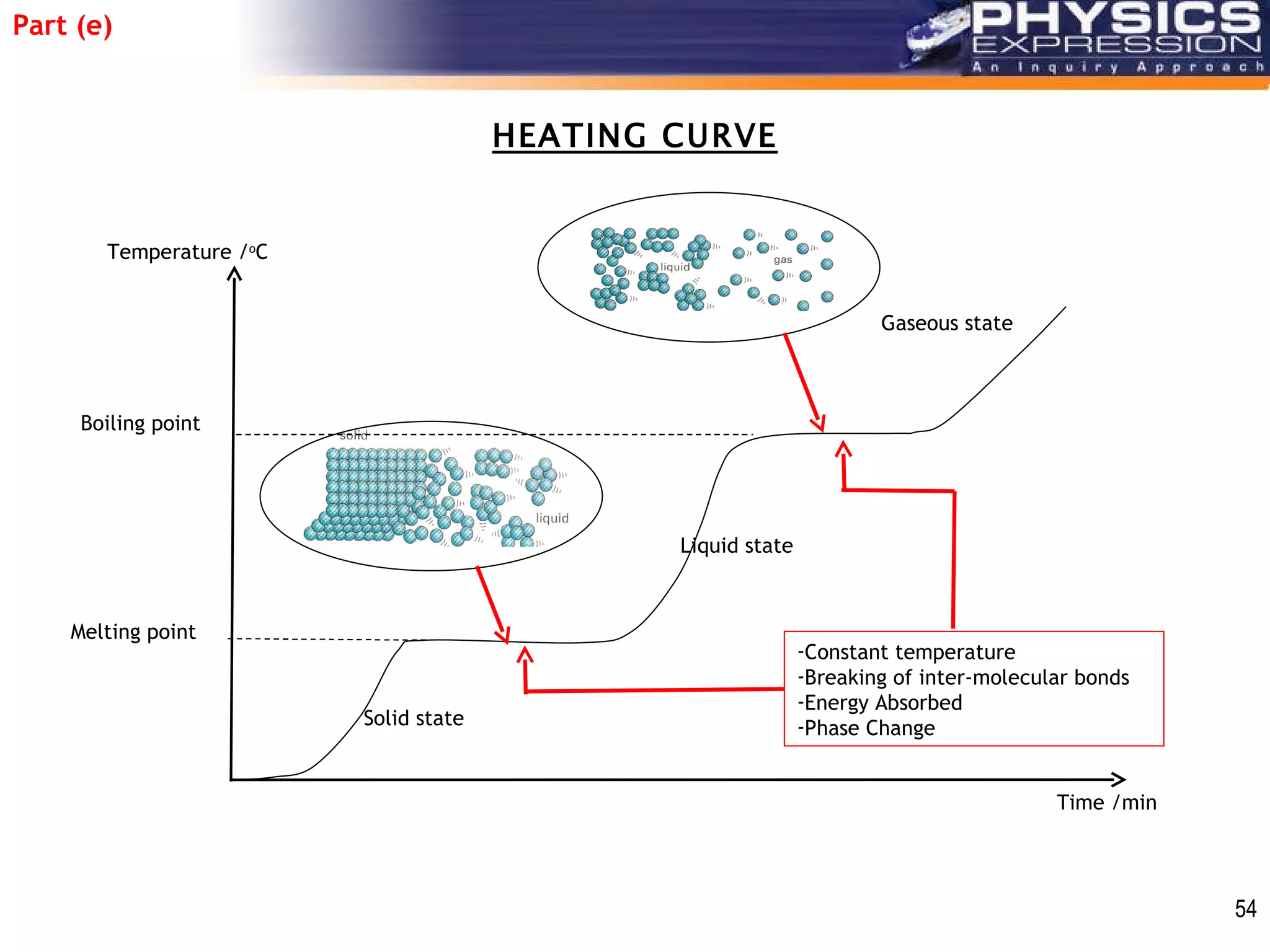
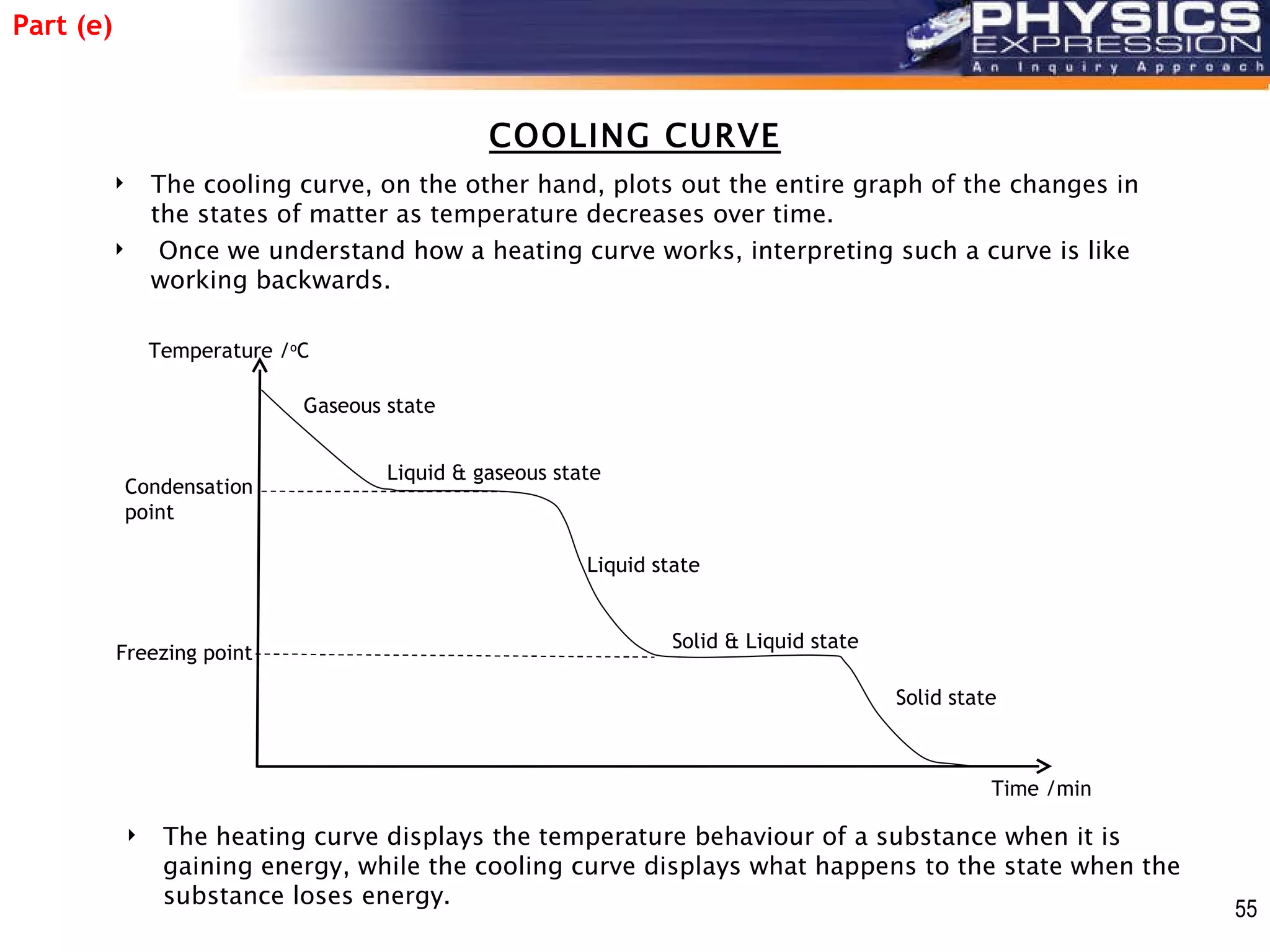
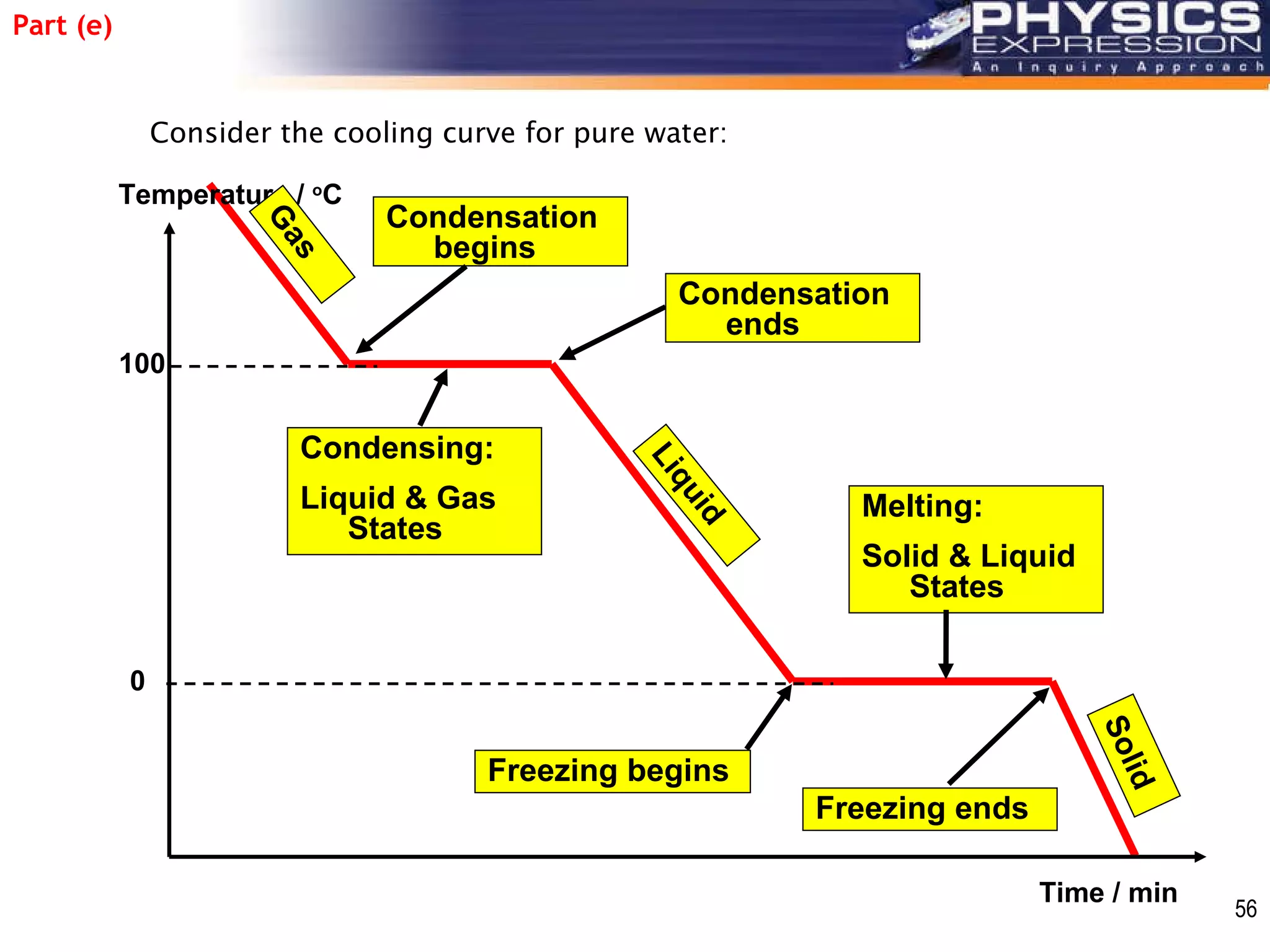
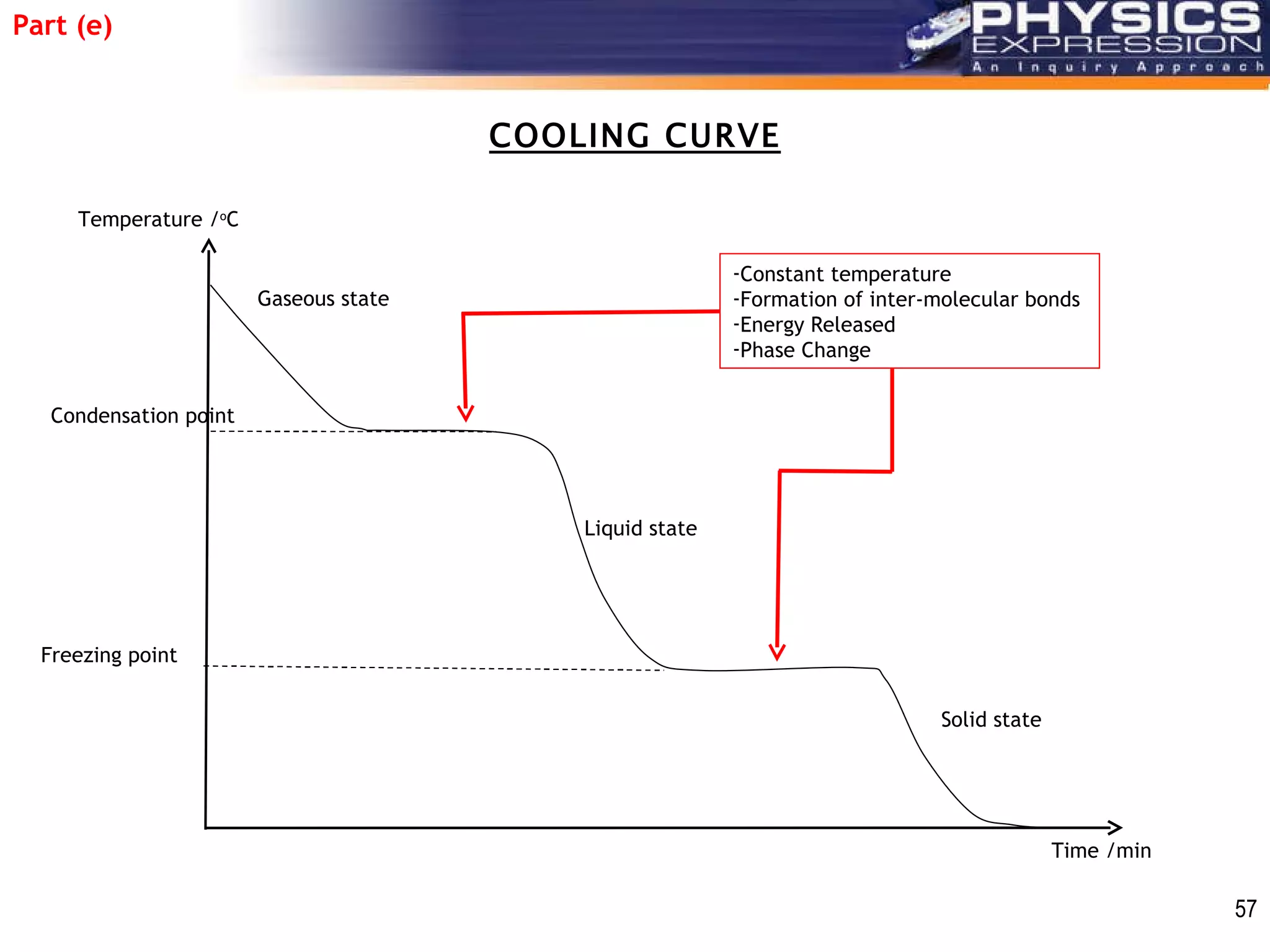
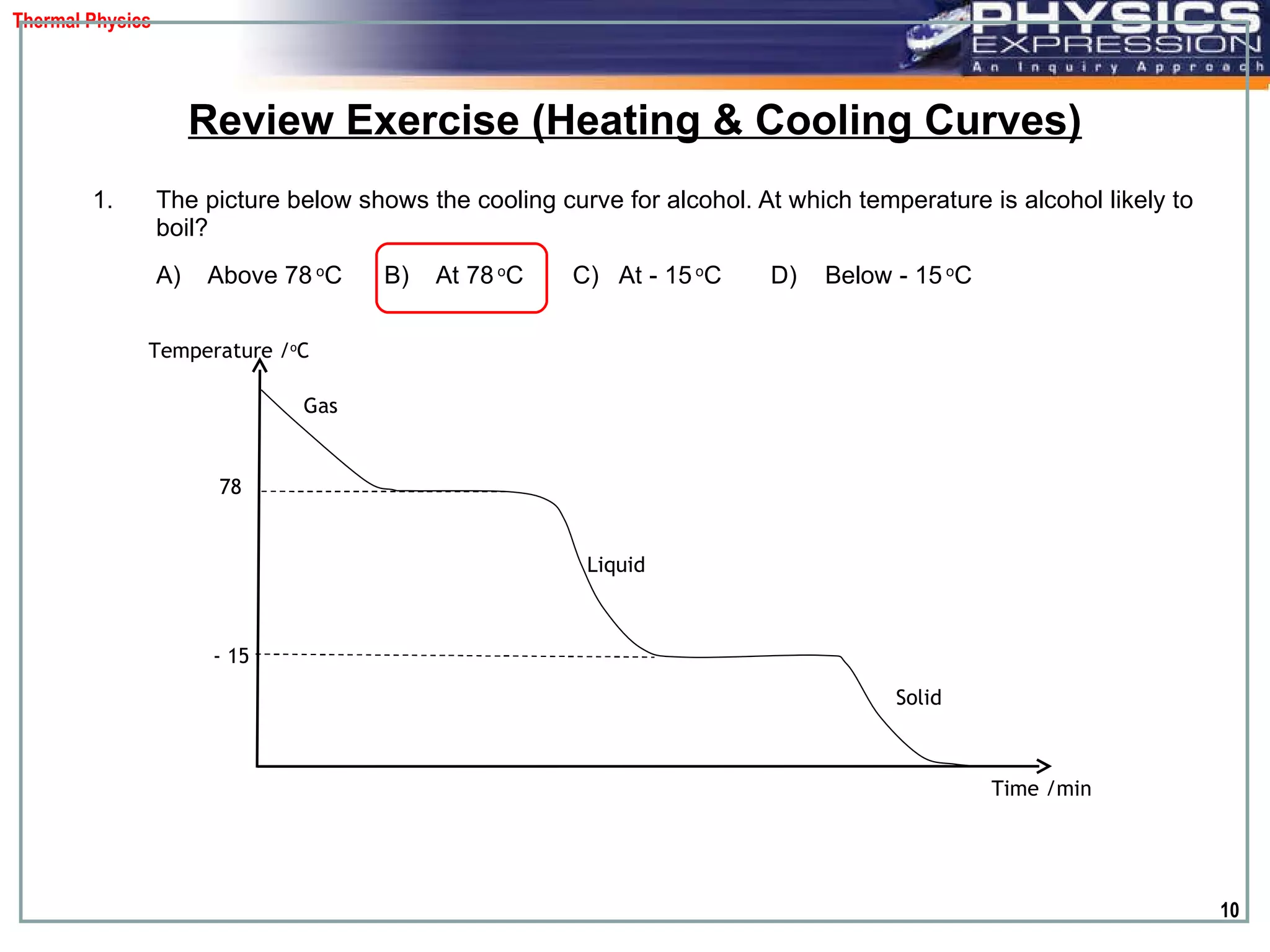
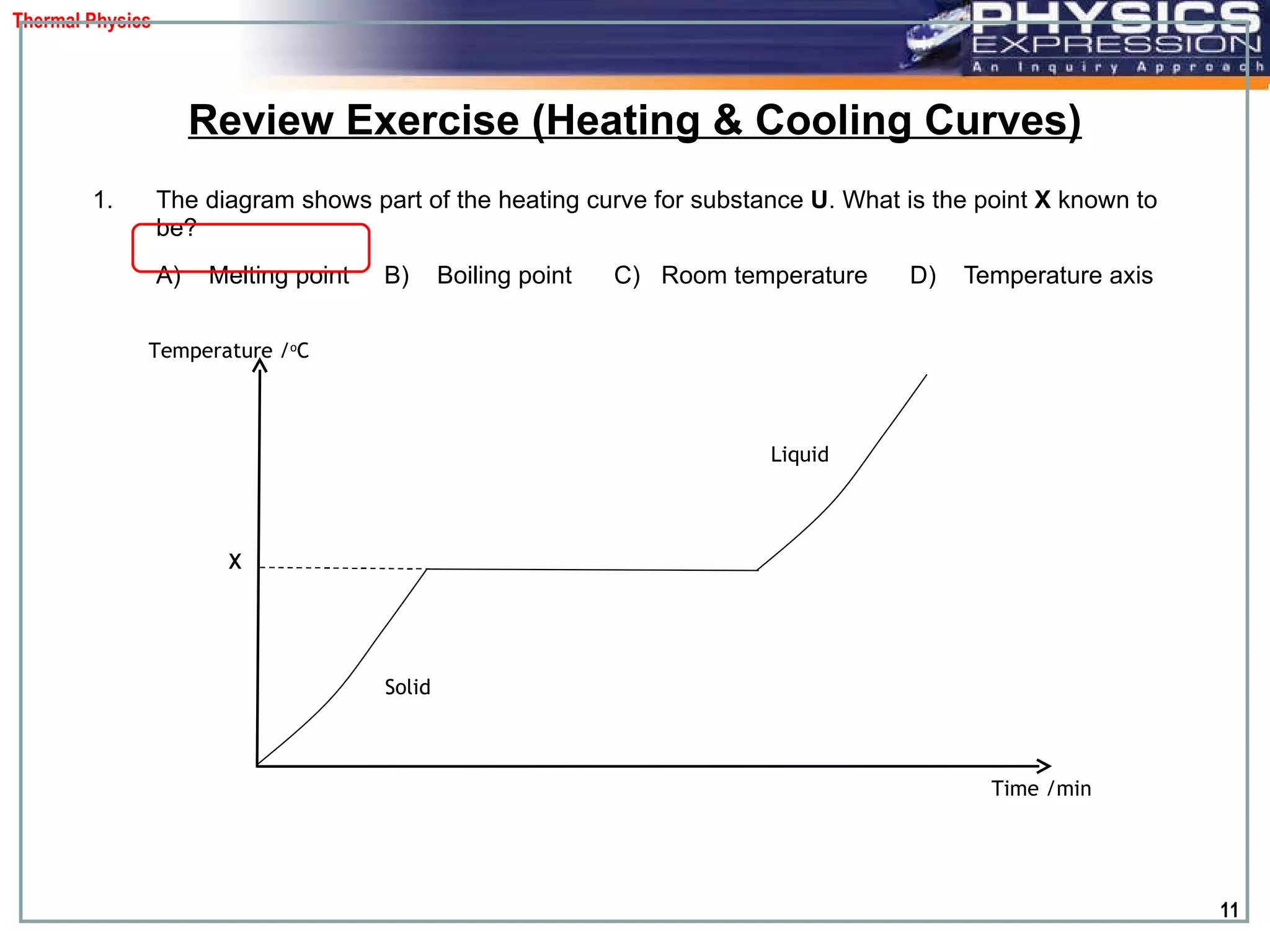
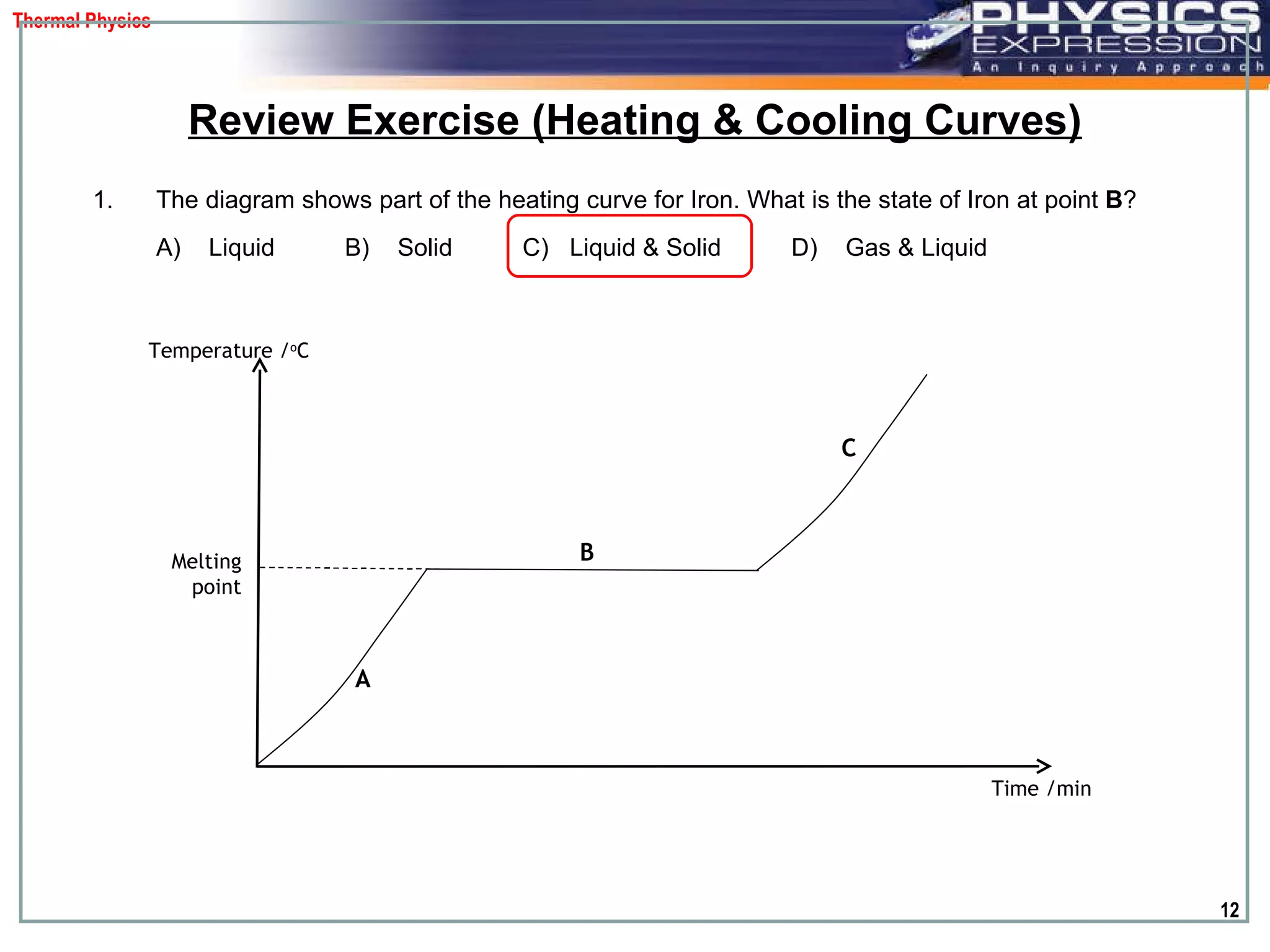
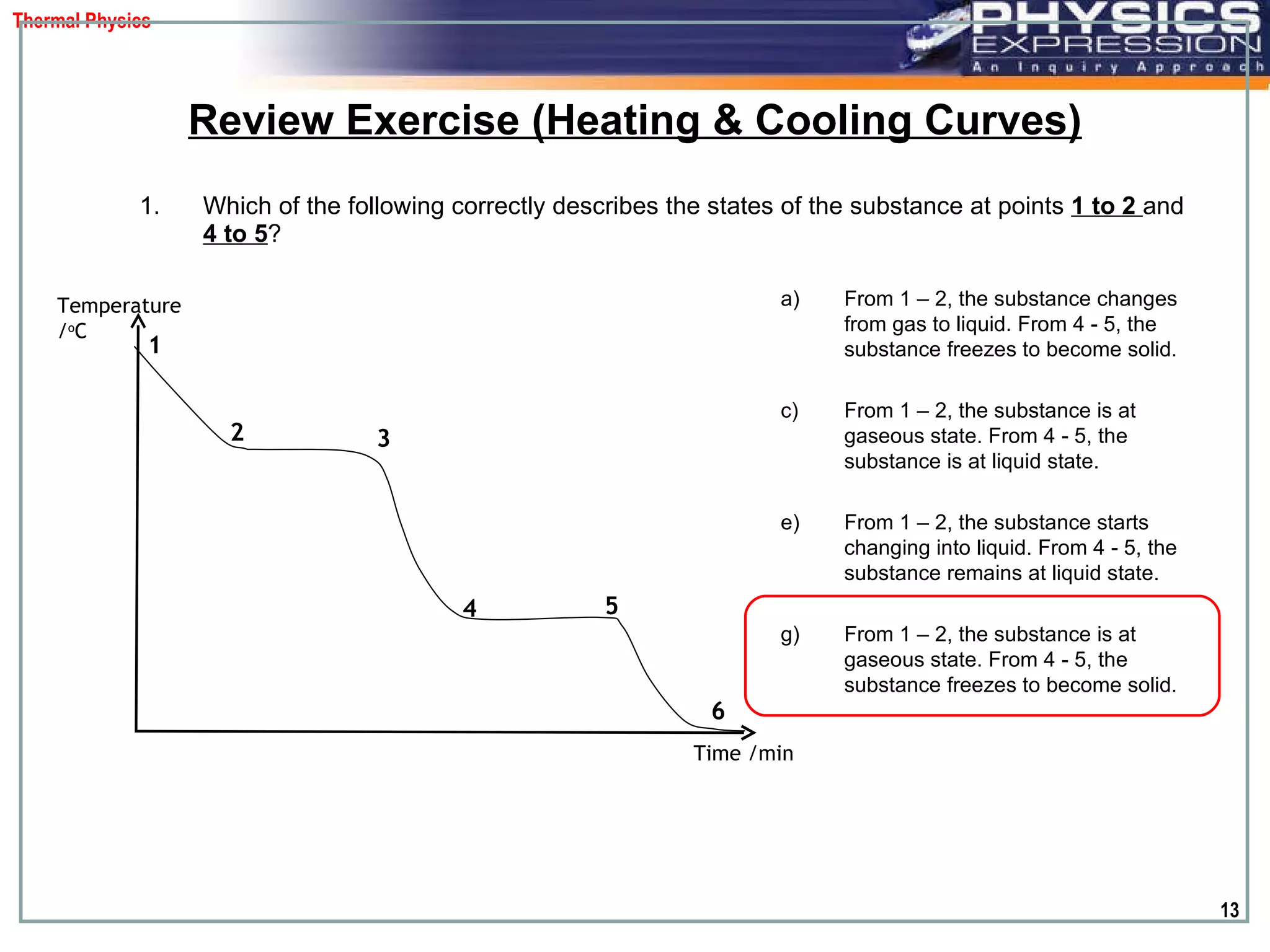
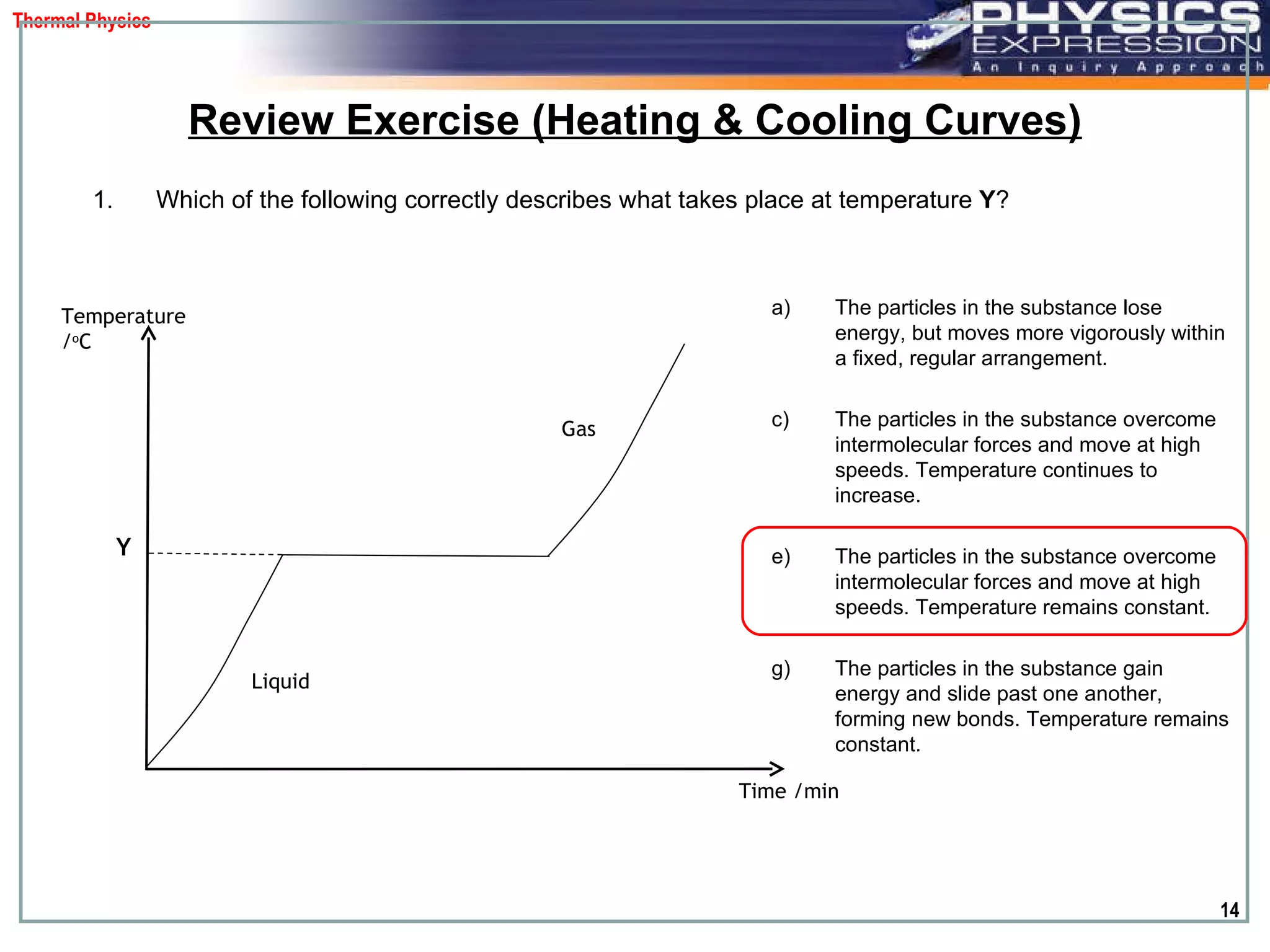
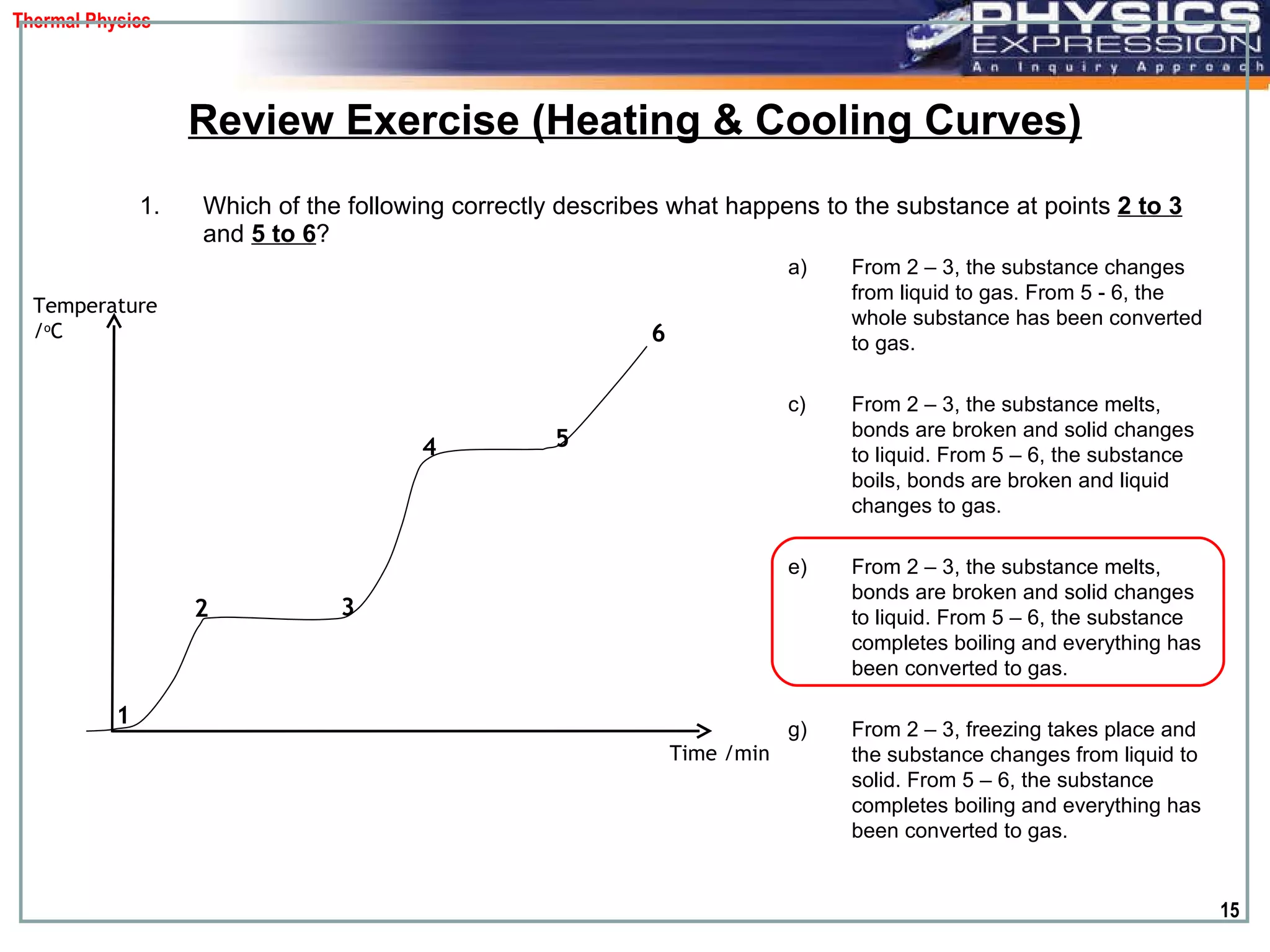
The document discusses heating and cooling curves. It explains that a heating curve plots the changes in a substance's state as temperature increases over time, identifying fixed points like melting and boiling points. A cooling curve similarly plots changes as temperature decreases. The curves allow identification of a substance's state at a given temperature or time. Examples of heating and cooling curves for water and alcohol are provided.