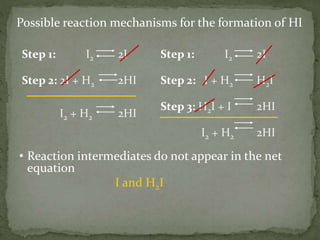
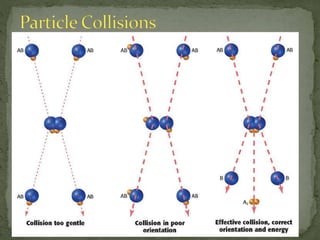
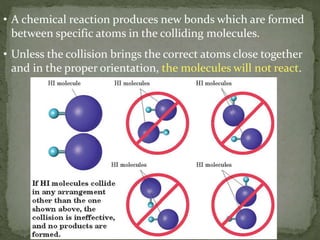
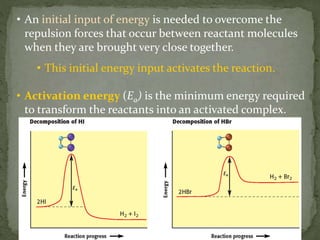
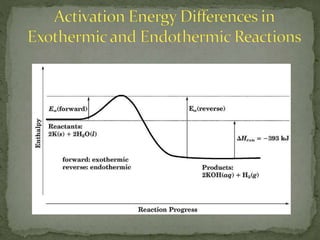
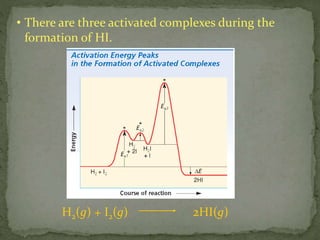
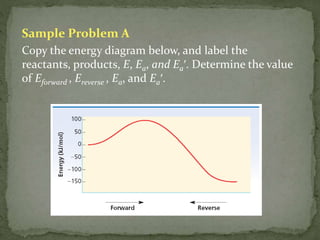
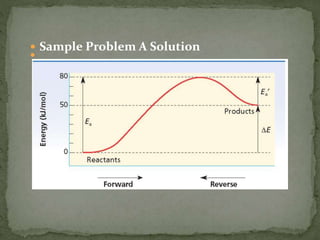
The document discusses reaction mechanisms and collision theory. It defines key concepts like the activated complex, which is a short-lived transitional structure during a reaction where old bonds are breaking and new bonds forming. Activation energy is the minimum energy needed for molecules to collide and form the activated complex. Collision theory states that for a reaction to occur molecules must collide with enough kinetic energy to overcome repulsive forces and in the proper orientation for new bonds to form.