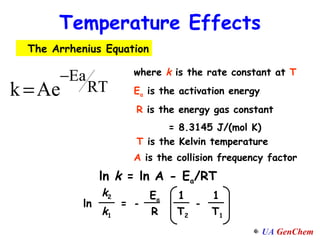
The document summarizes key concepts about the effects of temperature and catalysts on reaction rates:
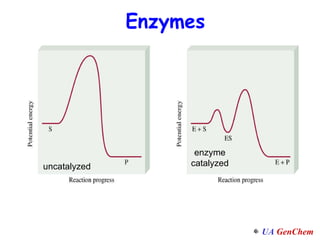
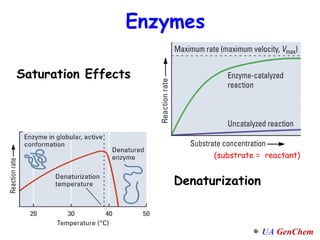
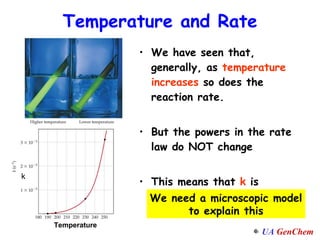
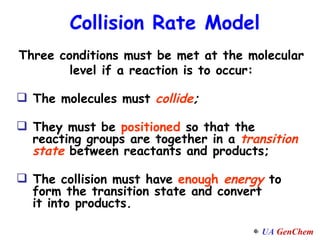
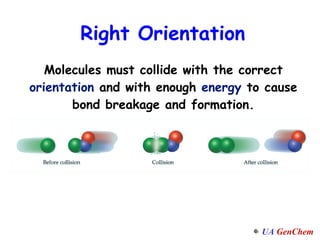
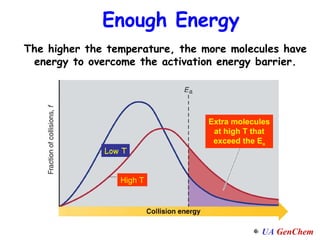
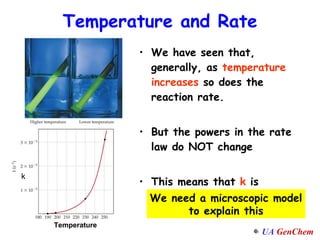
1) Increasing temperature generally increases reaction rate because more molecules have enough energy to overcome the activation energy barrier.
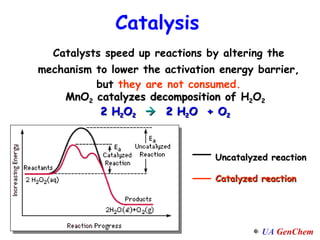
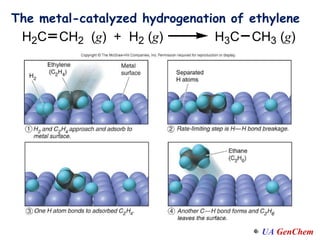
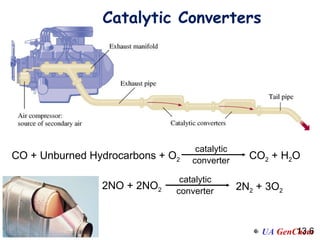
2) Catalysts speed up reactions by lowering the activation energy needed, allowing reactions to occur more quickly via a different mechanism.
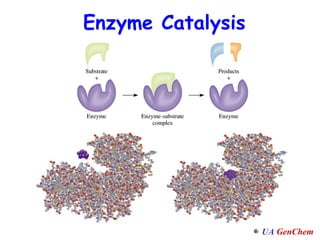
3) Enzymes are biological catalysts that regulate metabolic reactions in living organisms and act to reduce activation energies.




![Series of plots of concentra-tion vs. time Initial rates Reaction orders Rate constant ( k ) and actual rate law Integrated rate law (half-life, t 1/2 ) Rate constant and reaction order Activation energy, E a Plots of concentration vs. time Overview Find k at varied T Determine slope of tangent at t 0 for each plot Compare initial rates when [A] changes and [B] is held constant and vice versa Substitute initial rates, orders, and concentrations into general rate law: rate = k [A] m [B] n Use direct, ln or inverse plot to find order Rearrange to linear form and graph Find k at varied T](https://image.slidesharecdn.com/lectw3152d2-arrheniusandcatalystsalg1-110302112548-phpapp02/85/Lect-w3-152_d2-arrhenius-and-catalysts_alg-1-21-320.jpg)