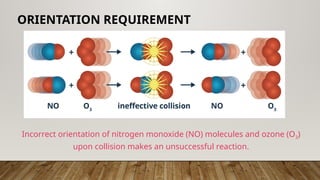
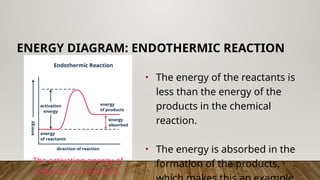
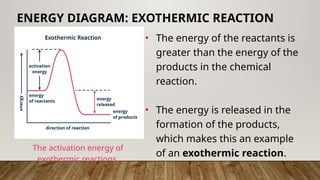
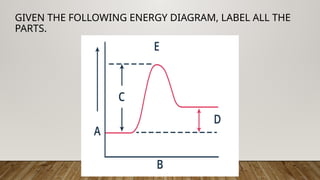
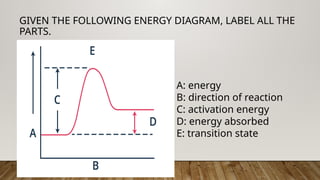
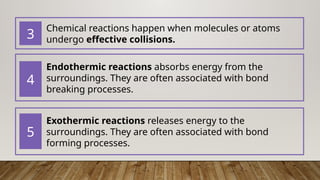
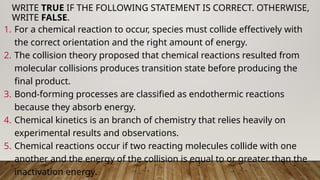
The document outlines the collision theory and chemical kinetics, which describe the mechanisms and rates of chemical reactions. It highlights that successful reactions require proper collisions with correct orientation and sufficient energy, defined as activation energy. Additionally, it contrasts endothermic and exothermic reactions, and introduces the transition state theory, emphasizing the intermediary steps in chemical reactions.