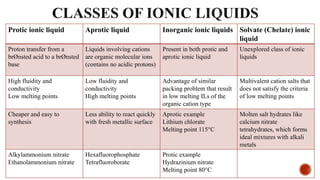
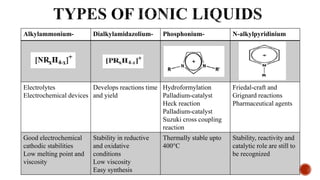
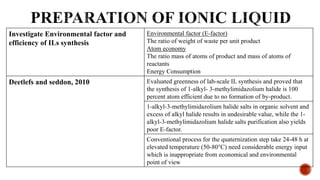
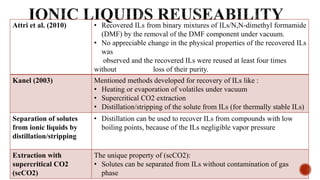
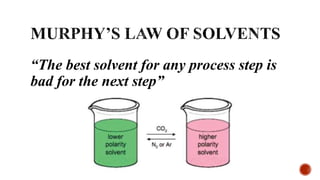
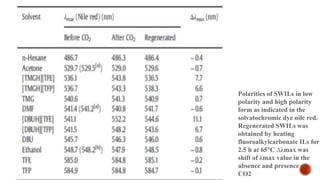
The document discusses ionic liquids, which are organic salts that are liquid below 100°C. They can be used as solvents in various applications such as electrochemical devices and chemical synthesis. The document outlines the history of ionic liquids and different types including protic and aprotic ionic liquids. It also discusses the use of ionic liquids in applications like electrolytes and catalysis. Furthermore, it covers switchable ionic liquids that can change polarity and discusses their synthesis and potential to reduce solvent use. The document emphasizes the need to consider the full life cycle and disposal of ionic liquids to determine their sustainability.




![bmpy][N(Tf)2 : 1-butyl-3-methylpyridinium bis(trifluoromethylsulfonyl)imide](https://image.slidesharecdn.com/ionicliquidwithswitchablepolarity-170404165727/85/Ionic-liquid-with-switchable-polarity-11-320.jpg)

![Synthesize of switchable ionic liquids (SWILs) using 2,2,2-Trifluoroethanol
(TFE;99%),2,2,3,3-tetrafluoro-1-propanol(TFP;99%),1,8-diazabicyclo-[5.4.0]-undec-
7-ene(DBU;98%),1,1,3,3-tetramethyguanidine(TMG;99%)and phenolphthalein (99%),
Nile Red (99.5%), analytical grade heptane and CO2.](https://image.slidesharecdn.com/ionicliquidwithswitchablepolarity-170404165727/85/Ionic-liquid-with-switchable-polarity-15-320.jpg)
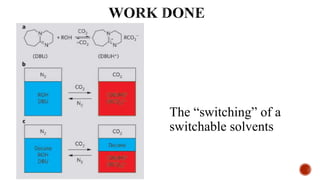
![Switchable polarity images of four switchable polarity of ionic liquids with a CO2
as the gas trigger: (a)[DBUH][TFE],(b)[TMGH][TFE],(c)[DBUH][TFP], and
(d) [TMGH][TFP].](https://image.slidesharecdn.com/ionicliquidwithswitchablepolarity-170404165727/85/Ionic-liquid-with-switchable-polarity-16-320.jpg)

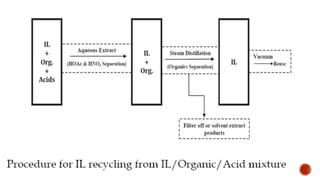
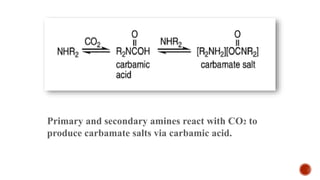
![Prepared as two-liquid component mixtures, either 1,8-
diazabicyclo-[5.4.0]-undec-7-ene DBU and an alcohol shown
in figure below or DBU and a primary amine](https://image.slidesharecdn.com/ionicliquidwithswitchablepolarity-170404165727/85/Ionic-liquid-with-switchable-polarity-19-320.jpg)

![ Abu-eishah, S.I., 2010. Ionic Liquids Recycling for Reuse. Ion. Liq. - Classes Prop. 239–272.
doi:10.5772/853
Austen Angell, C., Ansari, Y., Zhao, Z., 2012. Ionic Liquids: Past, present and future. Faraday Discuss.
154, 9–27. doi:10.1039/C1FD00112D
Chen, Y., Cao, Y., Shi, Y., Xue, Z., Mu, T., 2012. Quantitative research on the vaporization and
decomposition of [EMIM][Tf 2N] by thermogravimetric analysis-mass spectrometry. Ind. Eng. Chem.
Res. 51, 7418–7427.
Cvjetko Bubalo, M., Radošević, K., Radojčić Redovniković, I., Halambek, J., Gaurina Srček, V., 2014. A
brief overview of the potential environmental hazards of ionic liquids. Ecotoxicol. Environ. Saf. 99, 1–
12. doi:10.1016/j.ecoenv.2013.10.019
Docherty, K.M., Dixon, J.K., Kulpa, C.F., 2007. Biodegradability of imidazolium and pyridinium ionic
liquids by an activated sludge microbial community. Biodegradation 18, 481–493. doi:10.1007/s10532-
006-9081-7
Ghandi, K., 2014. A Review of Ionic Liquids , Their Limits and Applications. Green Sustain. Chem. 4,
44–53. doi:10.4236/gsc.2014.41008](https://image.slidesharecdn.com/ionicliquidwithswitchablepolarity-170404165727/85/Ionic-liquid-with-switchable-polarity-23-320.jpg)


