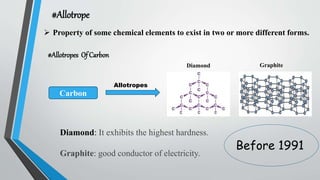
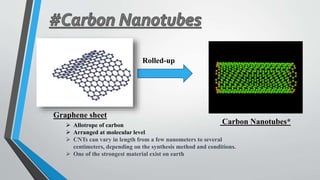
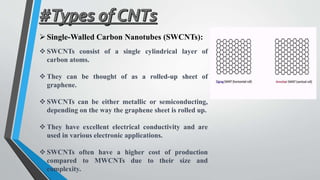
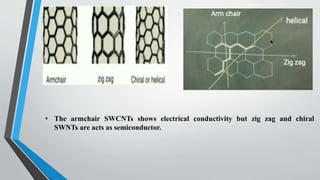
This document provides an overview of carbon nanotubes, including their discovery, types, synthesis methods, properties, applications, and future potential. It describes how carbon nanotubes are rolled-up graphene sheets that can be single-walled or multi-walled. The three main synthesis methods covered are laser ablation, arc discharge, and chemical vapor deposition. Key properties discussed include their strength, conductivity, flexibility, and thermal properties. Applications mentioned span electronics, materials, sensors, batteries, and medical devices.