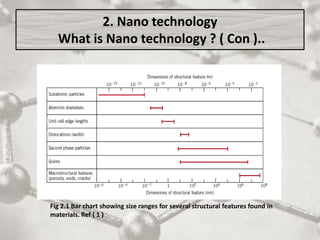
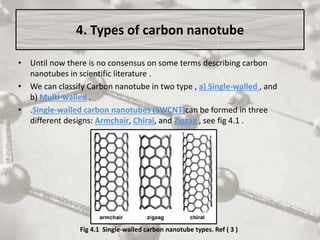
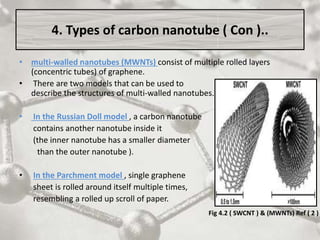
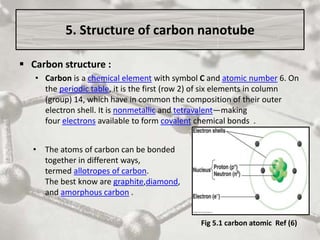
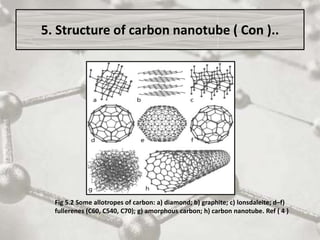
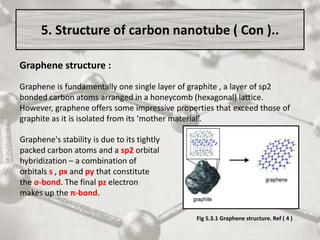
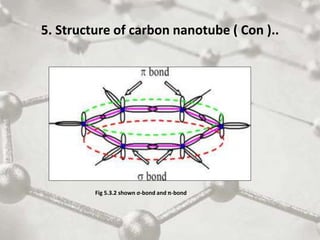
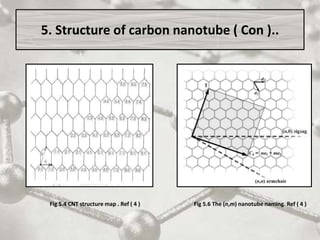
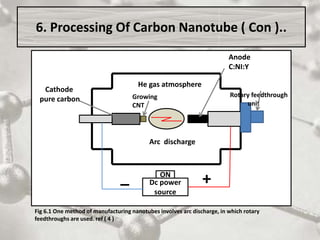
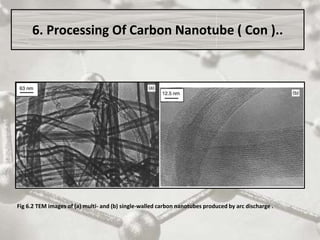
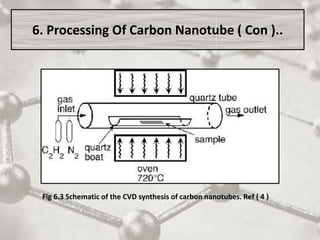
The document discusses carbon nanotubes, including their structure, types, and production methods. Carbon nanotubes are cylindrical tubes composed solely of carbon atoms. They can be single-walled or multi-walled, and have a diameter on the nanometer scale. Common production techniques include arc discharge, laser ablation, and chemical vapor deposition using a metal catalyst. Carbon nanotubes have exceptional mechanical and electrical properties and potential applications in materials, electronics, and biomedical fields.