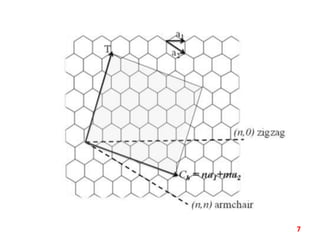
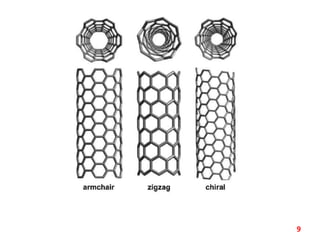
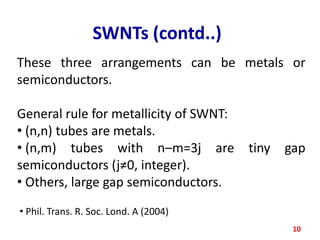
The document discusses carbon nanotubes (CNTs), their classification into single-walled (SWNTs) and multi-walled nanotubes (MWNTs), and their various synthesis methods, including arc discharge, laser ablation, and chemical vapor deposition. It highlights their properties such as strength, hardness, and electrical characteristics, along with potential applications in desalination, solar cells, ultracapacitors, and as light bulb filaments. Additionally, the document mentions other structures like nanobuds and nanotori that exhibit unique properties.