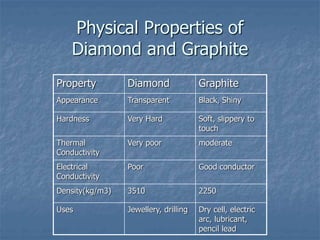
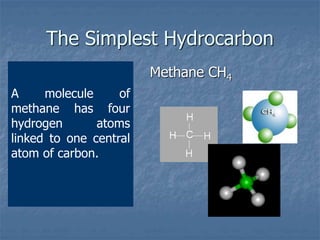
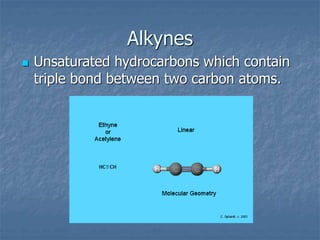
This document discusses carbon and its compounds. Carbon has four electrons in its outer orbit and can form four covalent bonds, giving it a valency of four. It is a non-metal that occurs naturally in two allotropes - diamond and graphite. While diamond and graphite are chemically identical, consisting entirely of carbon, they have very different physical properties due to differences in how the carbon atoms are arranged. Carbon has the unique ability to form long chains, providing a backbone for other atoms to attach to, resulting in the vast number and variety of carbon compounds found in nature, including hydrocarbons.