Embed presentation
Download to read offline
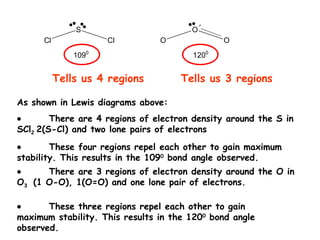
There are four regions of electron density around sulfur in SCl2 consisting of two sulfur-chlorine bonds and two lone pairs, which repel each other resulting in a 109 degree bond angle for maximum stability. Similarly, there are three regions around oxygen in O3 comprising one single bond, one double bond, and one lone pair, also repelling to achieve a 120 degree bond angle providing greatest stability.
