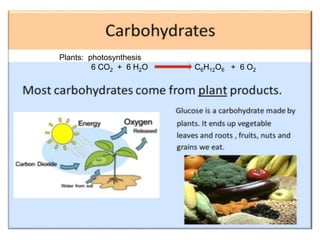
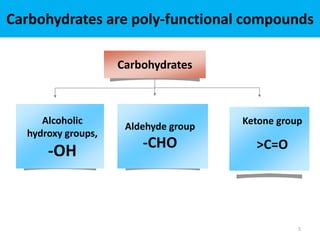
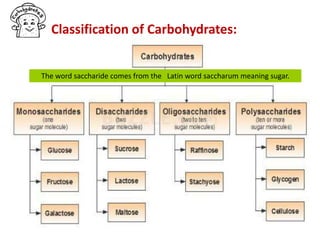
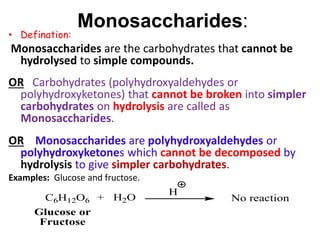
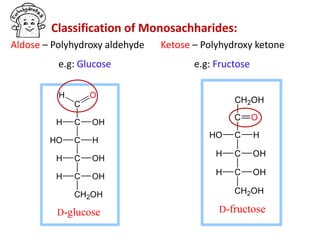
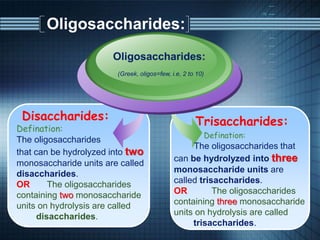
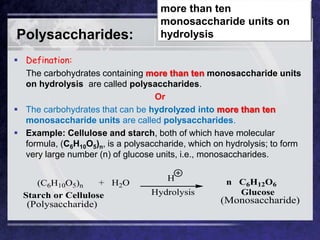
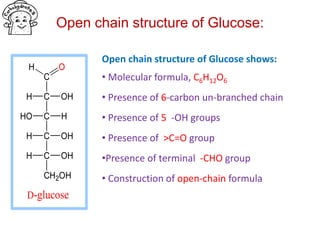
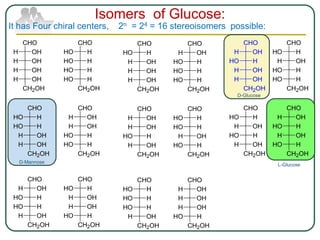
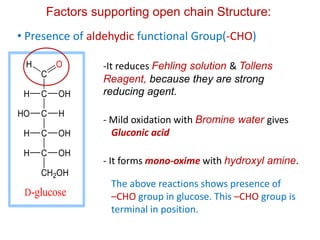
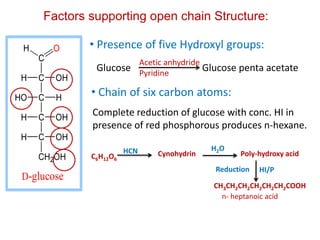
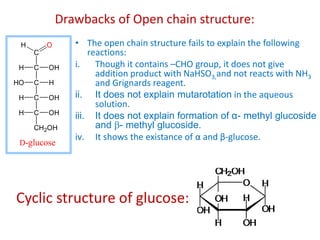
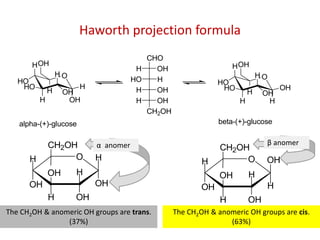
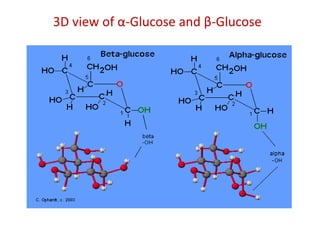
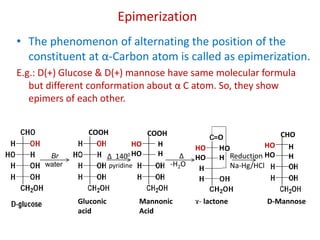
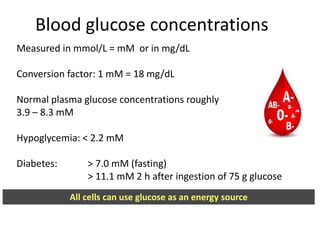
The document discusses carbohydrates, their classifications, structures, and functions. Carbohydrates, including monosaccharides, oligosaccharides, and polysaccharides, serve as key energy sources and structural components in biology. It also covers the open chain and cyclic structures of glucose, epimerization, and mutarotation, while highlighting glucose's role in providing energy for the body's cells.