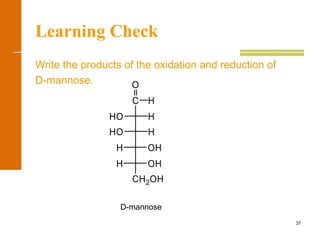
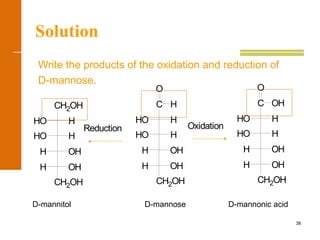
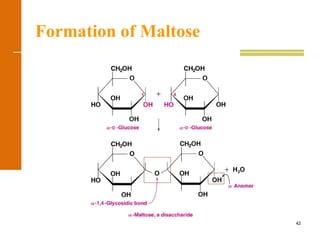
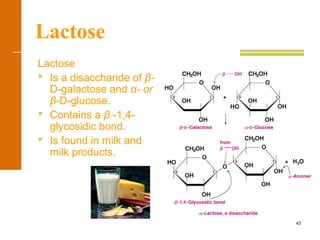
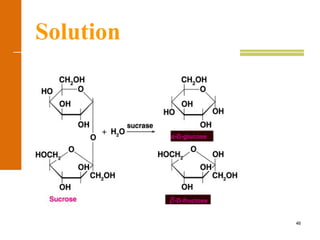
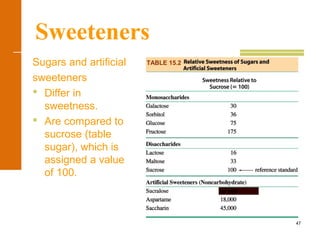
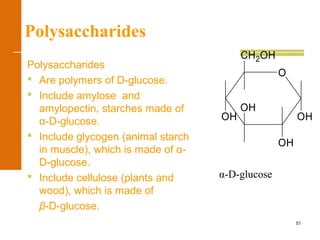
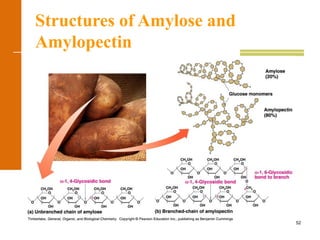
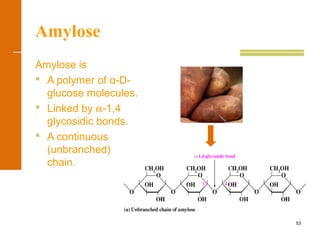
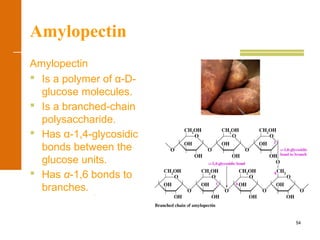
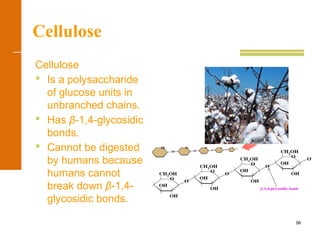
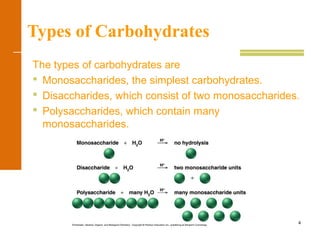
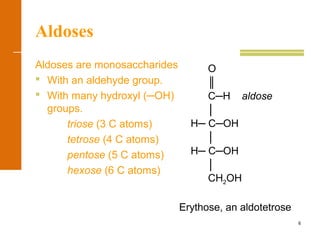
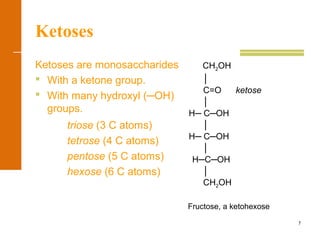
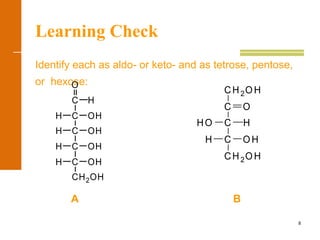
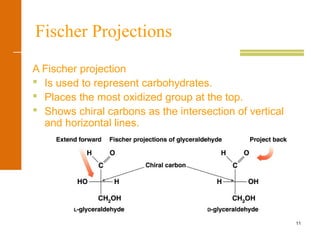
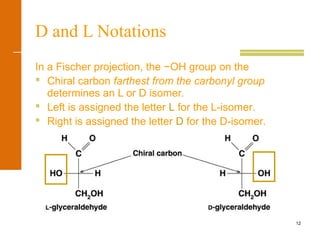
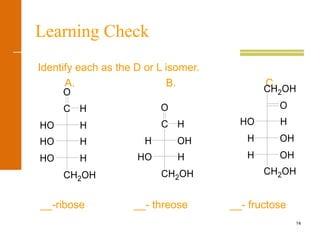
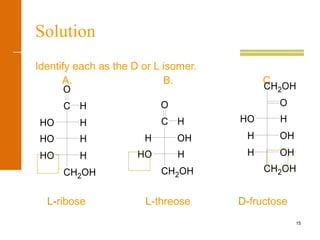
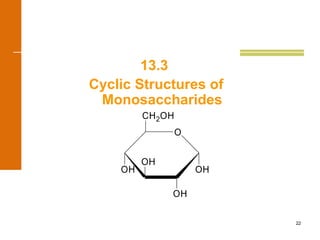
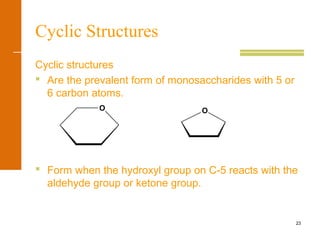
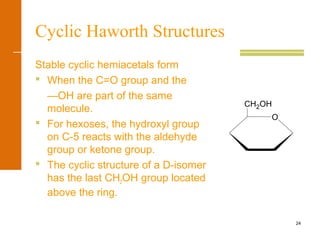
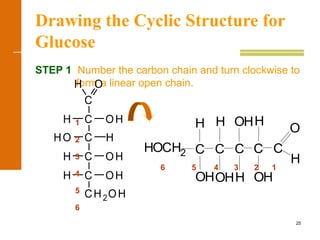
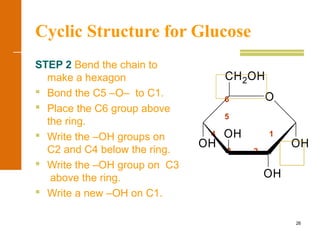
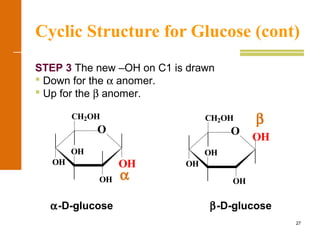
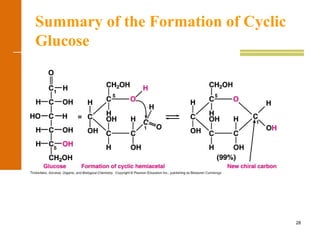
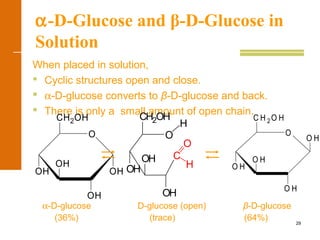
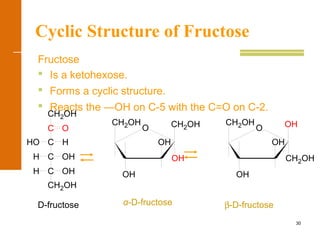
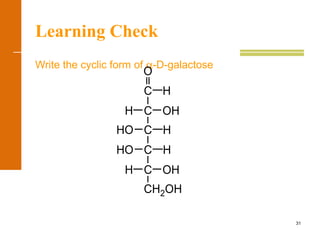
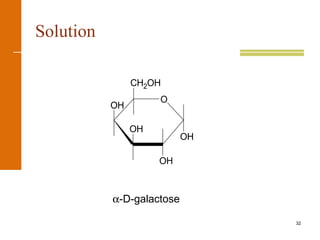
This document discusses carbohydrates. It defines carbohydrates and describes the main types: monosaccharides, disaccharides, and polysaccharides. It discusses important monosaccharides like glucose, fructose, and galactose. It describes their cyclic and linear structures using Fischer projections. It also covers properties of reducing sugars and examples of important disaccharides like maltose, lactose, and sucrose. Finally, it summarizes key polysaccharides like amylose, amylopectin, glycogen, and cellulose.










![35
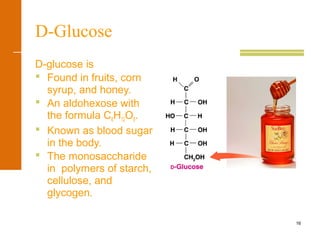
Oxidation of D-Glucose
+ Cu2O(s)
D-gluconic acidD-glucose
+ Cu2+
H OH
H OH
HHO
H OH
O
OH
CH2OH
C
C
C
C
C
H OH
H OH
HHO
H OH
O
H
CH2OH
C
C
C
C
C
[O]
Benedicts
reagent
Glucose is a reducing
sugar
Cu+
(reduced
form)
Glucose is oxidized
to a carboxylic acid](https://image.slidesharecdn.com/chapter-13carbohydrates-190219074804/85/Chapter-13-carbohydrates-35-320.jpg)