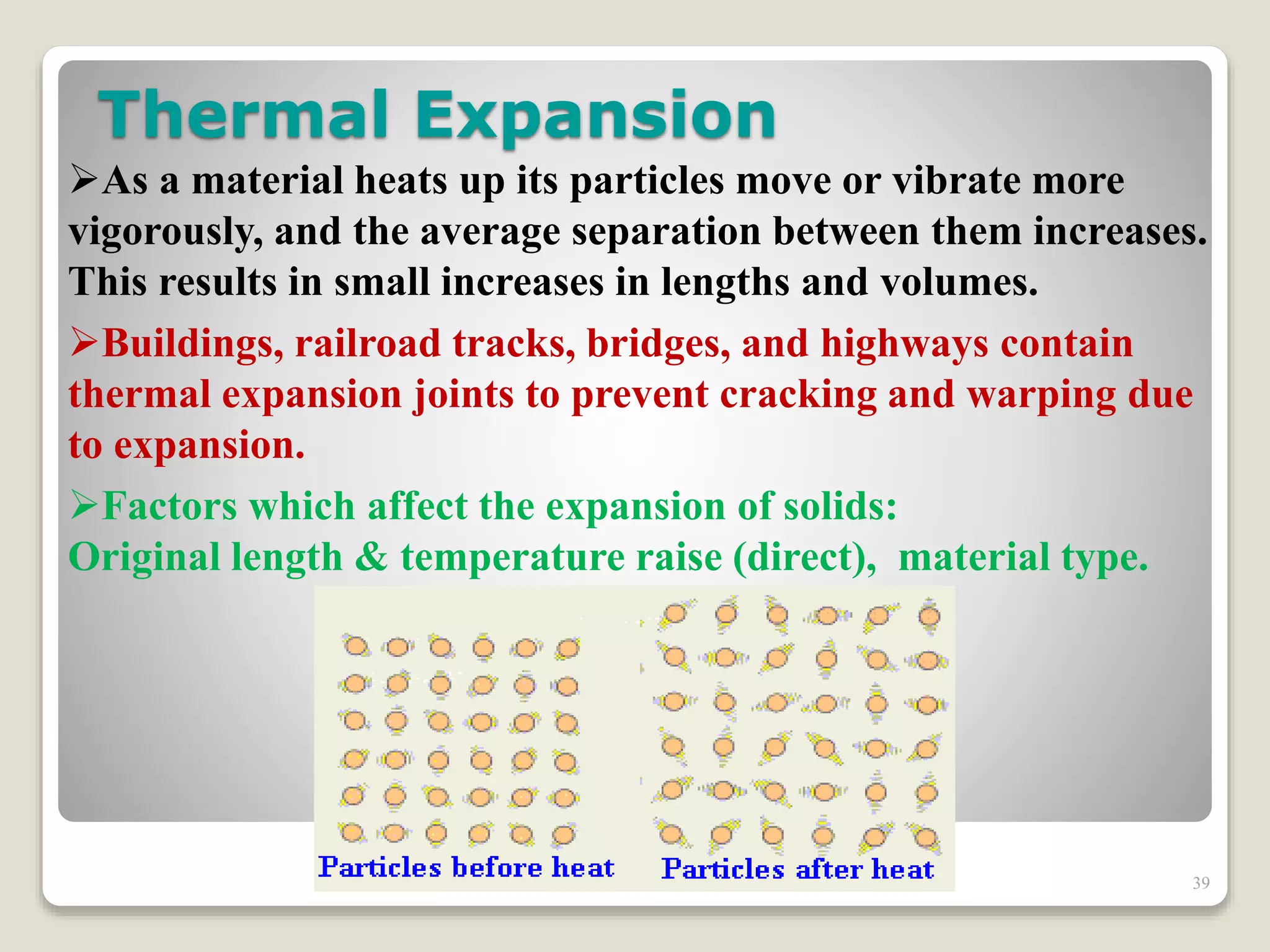
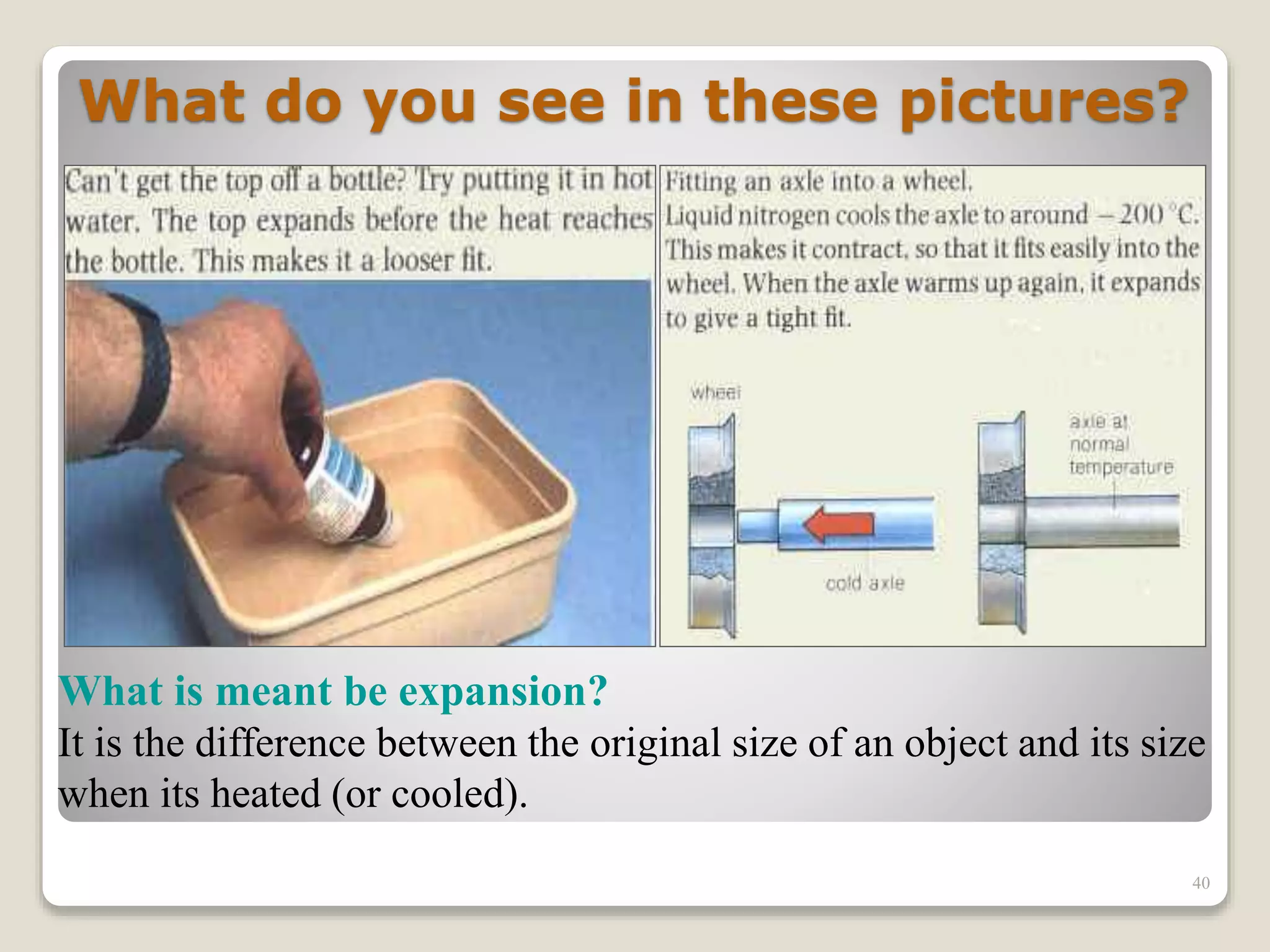
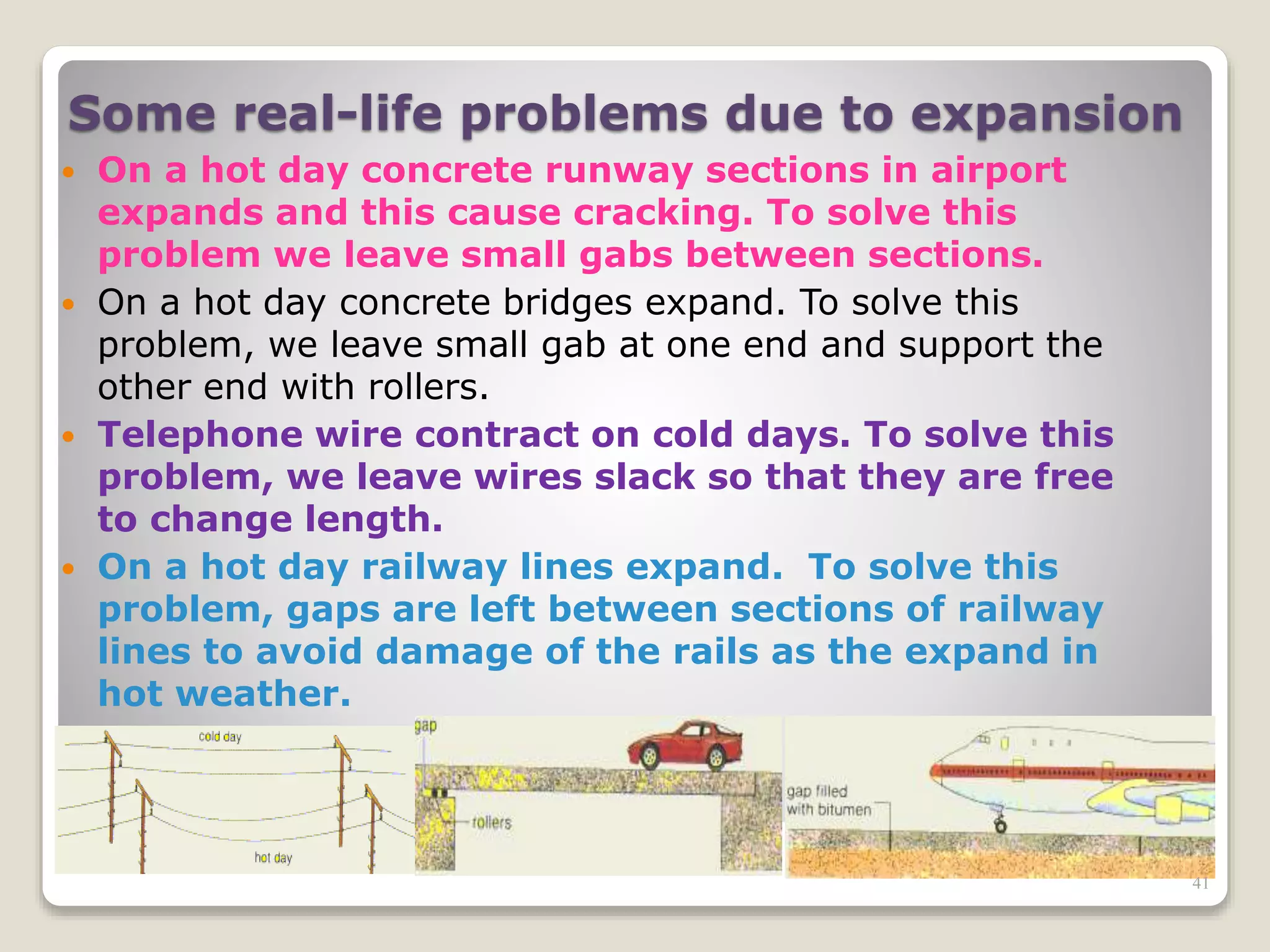
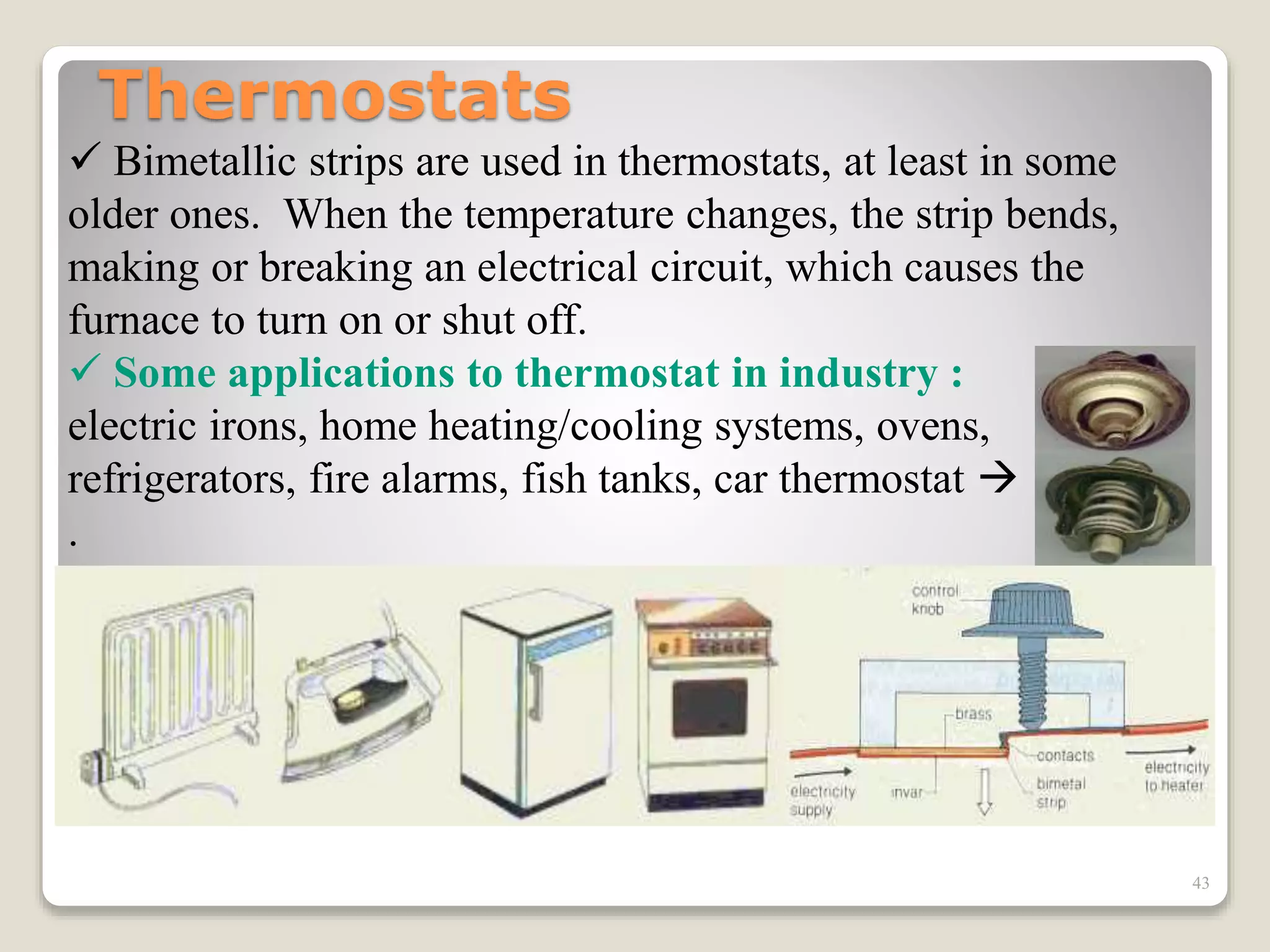
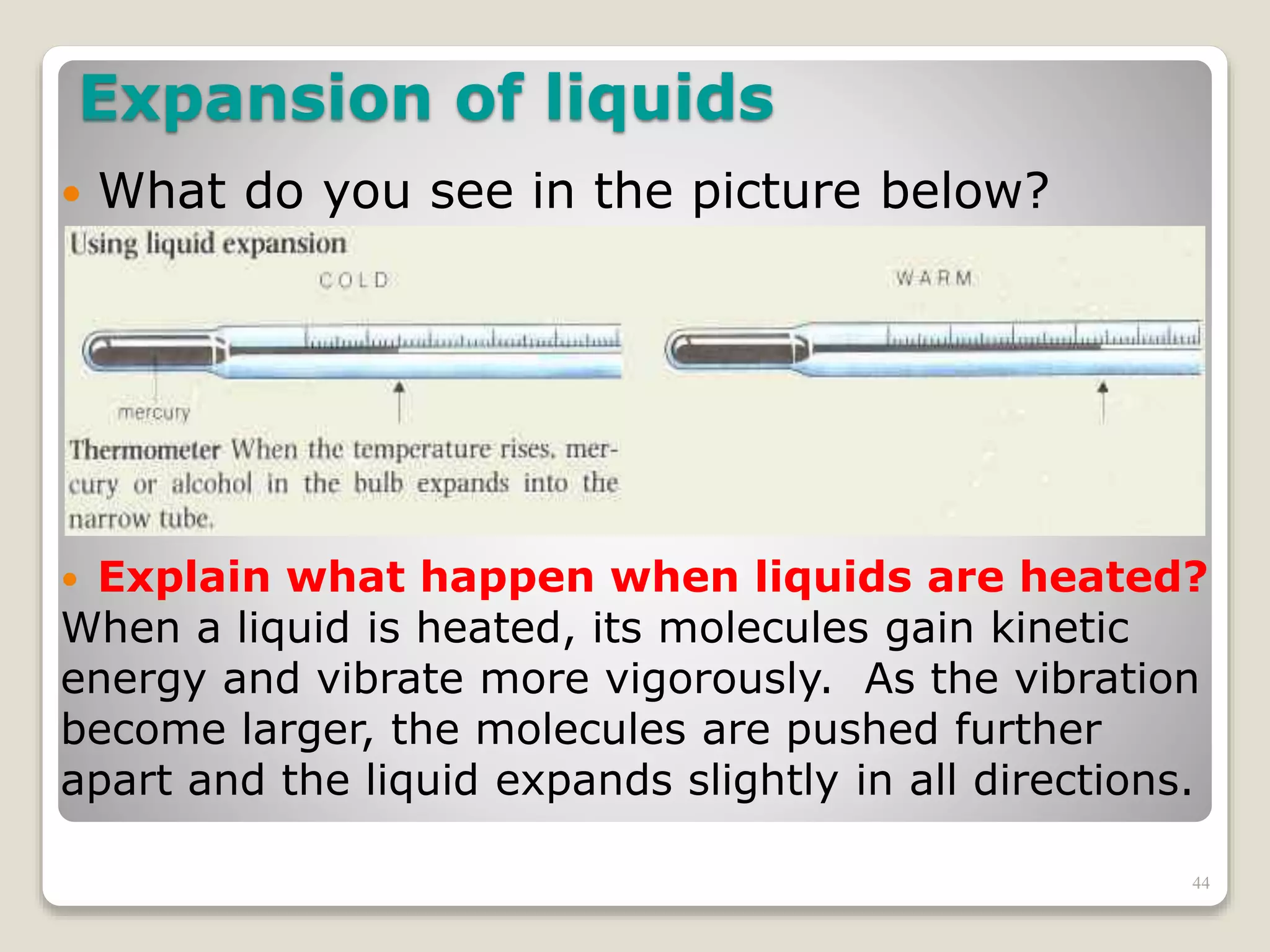
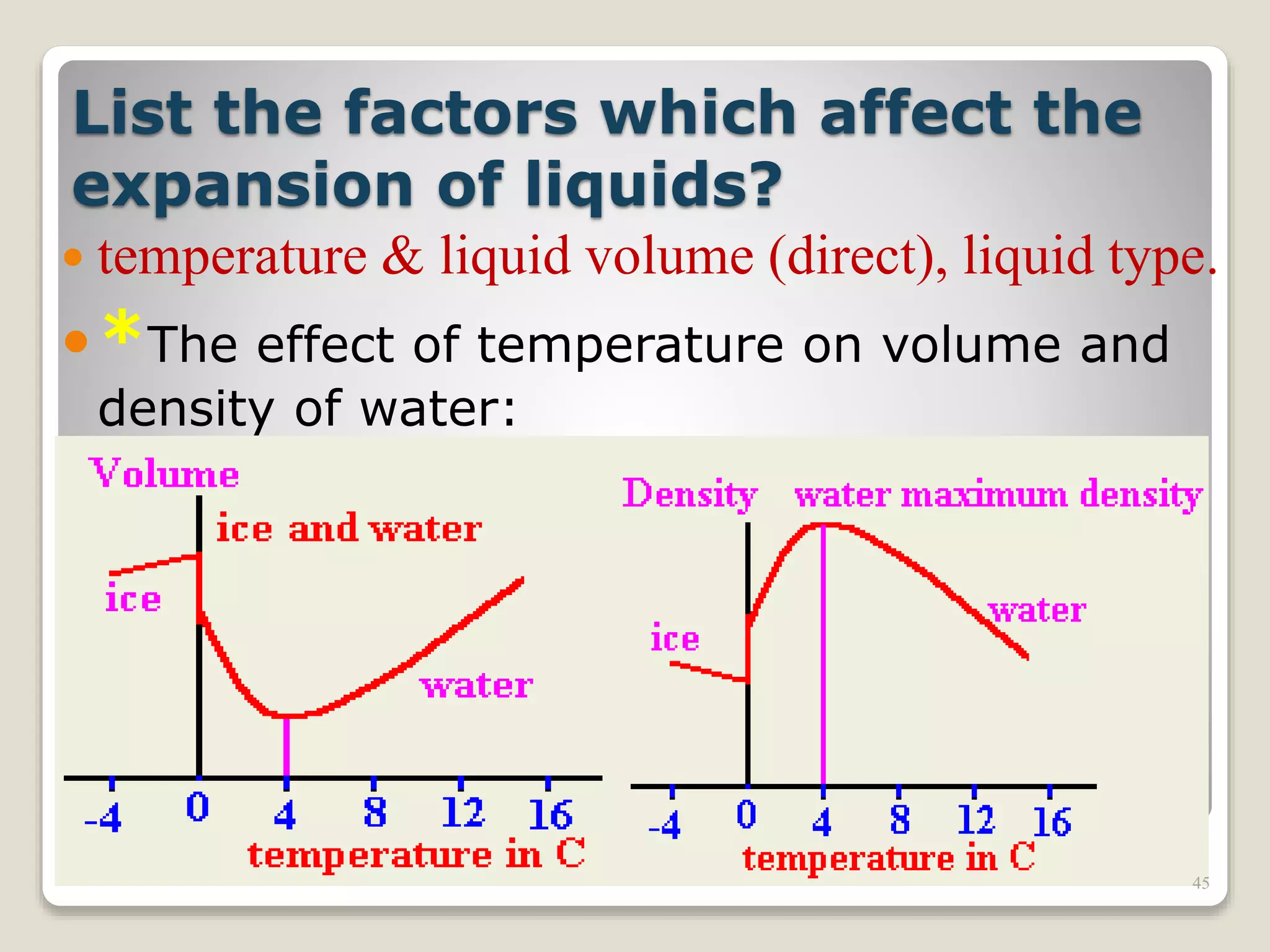
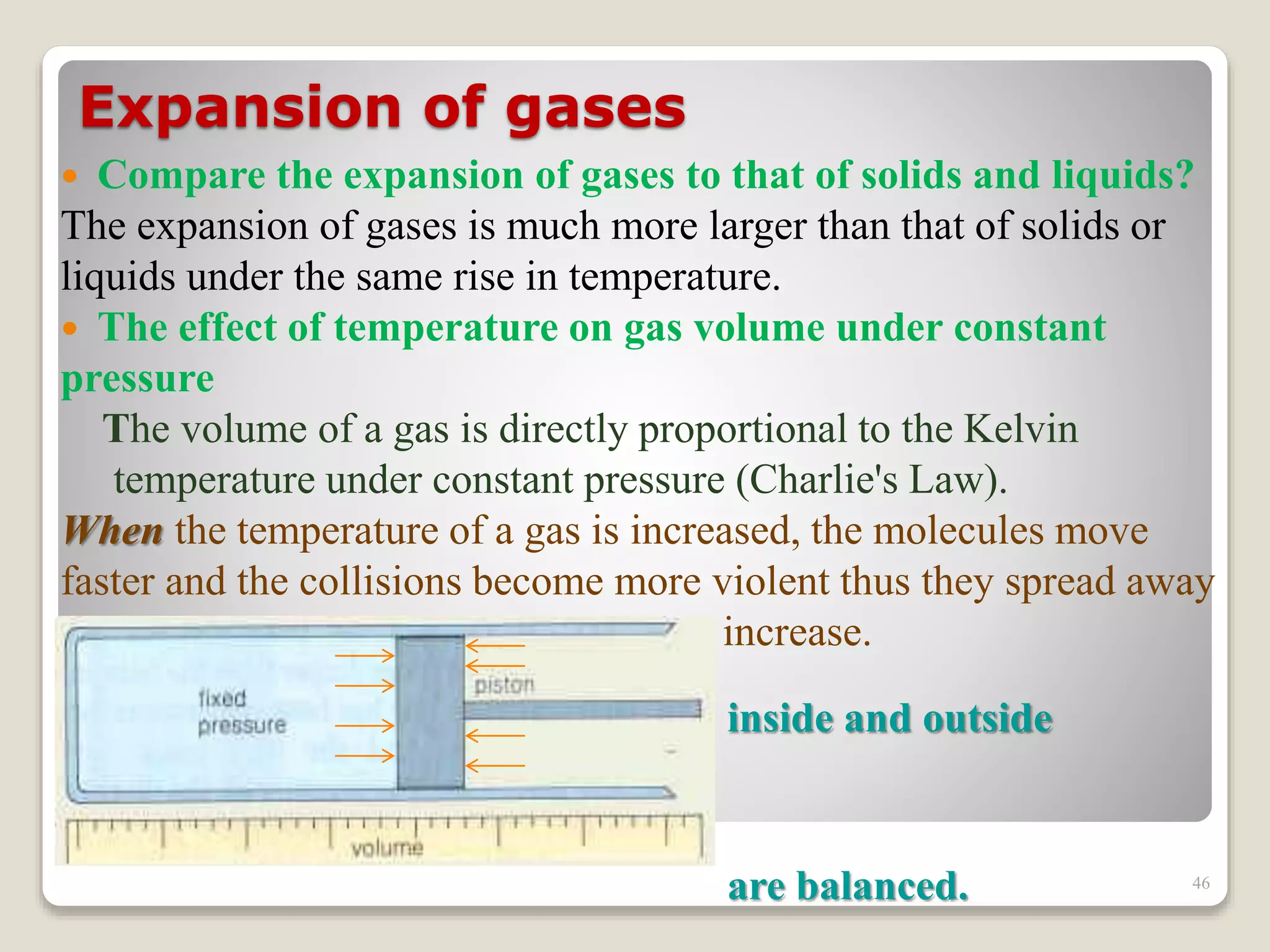
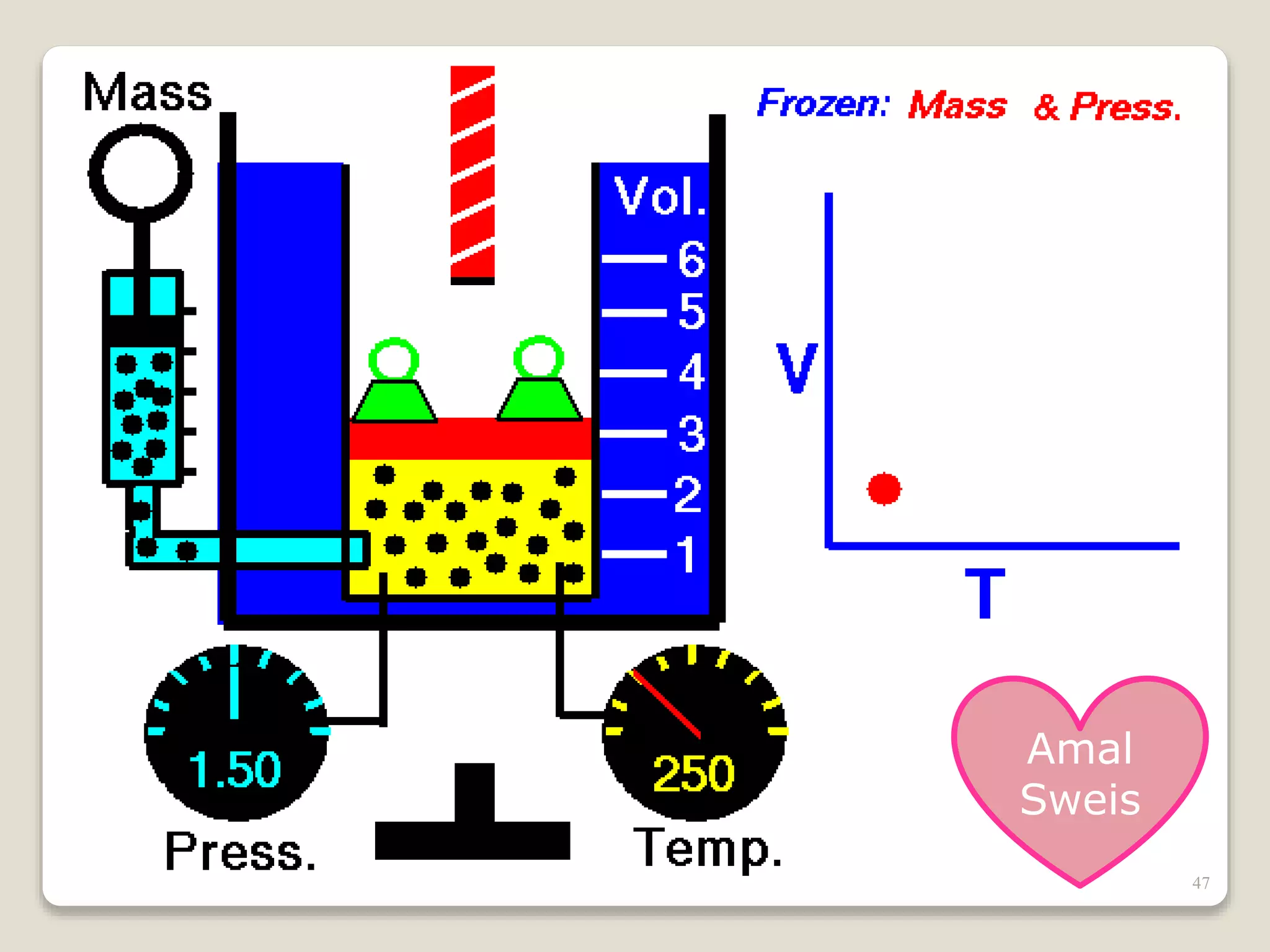
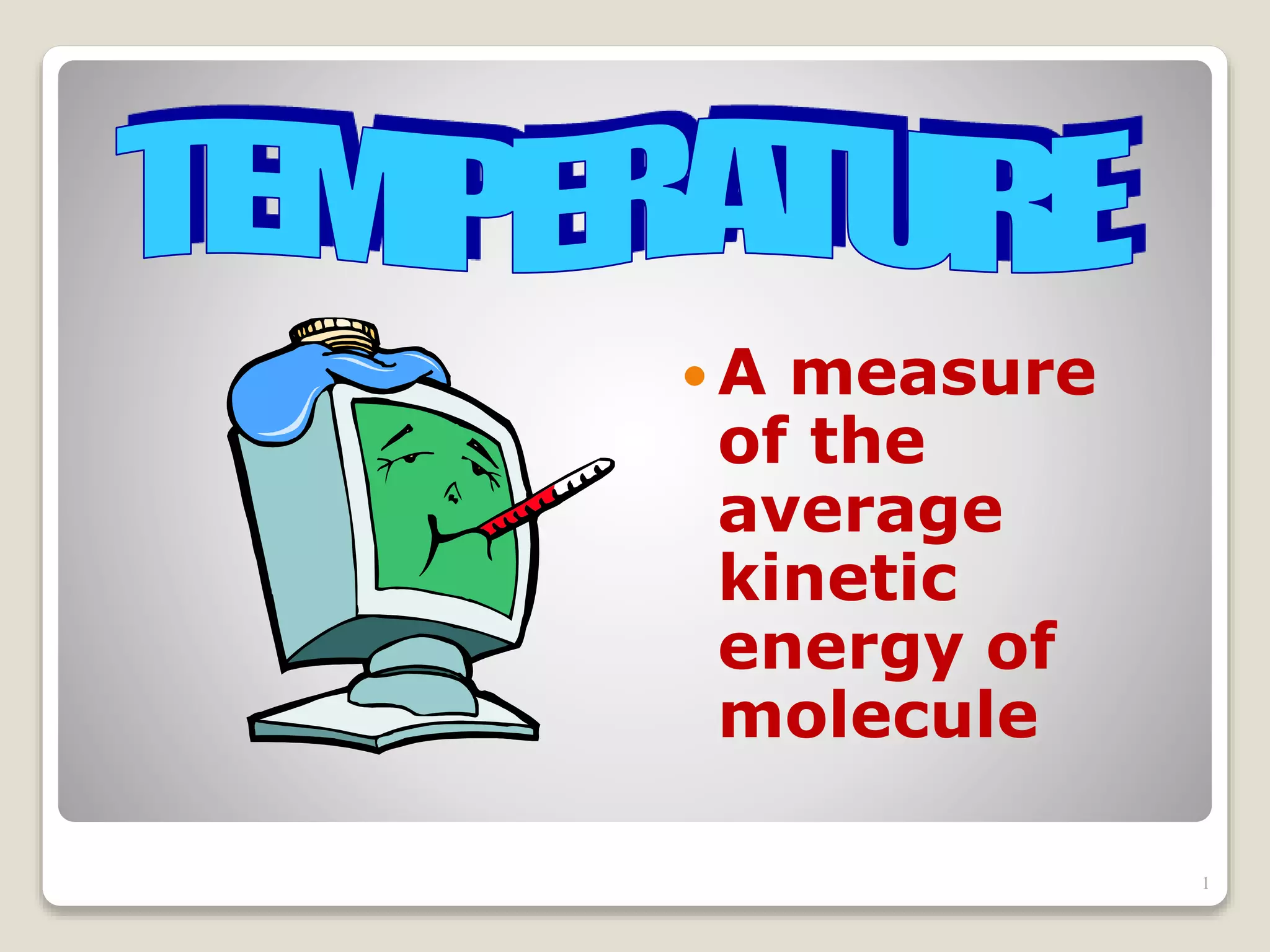
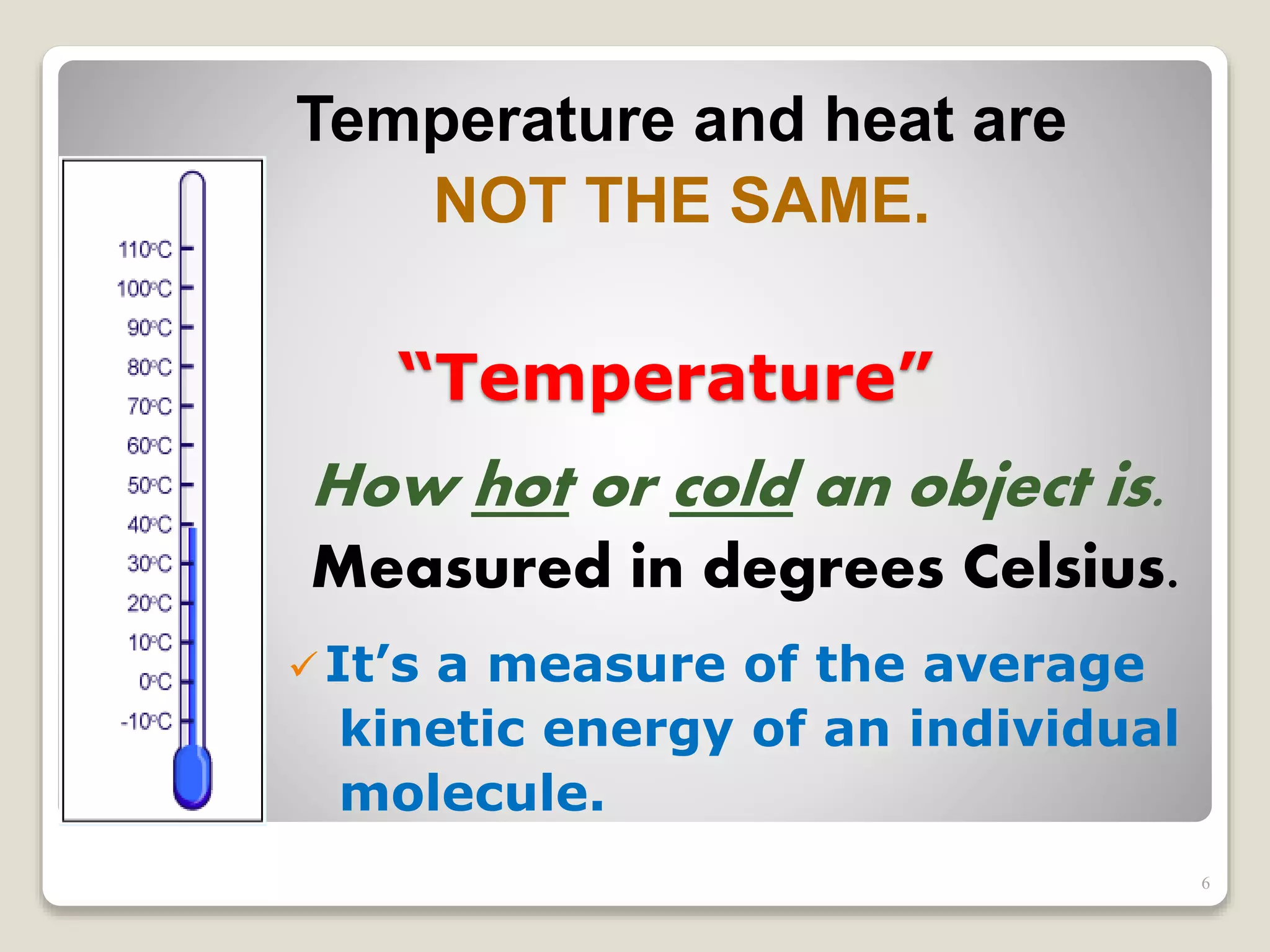
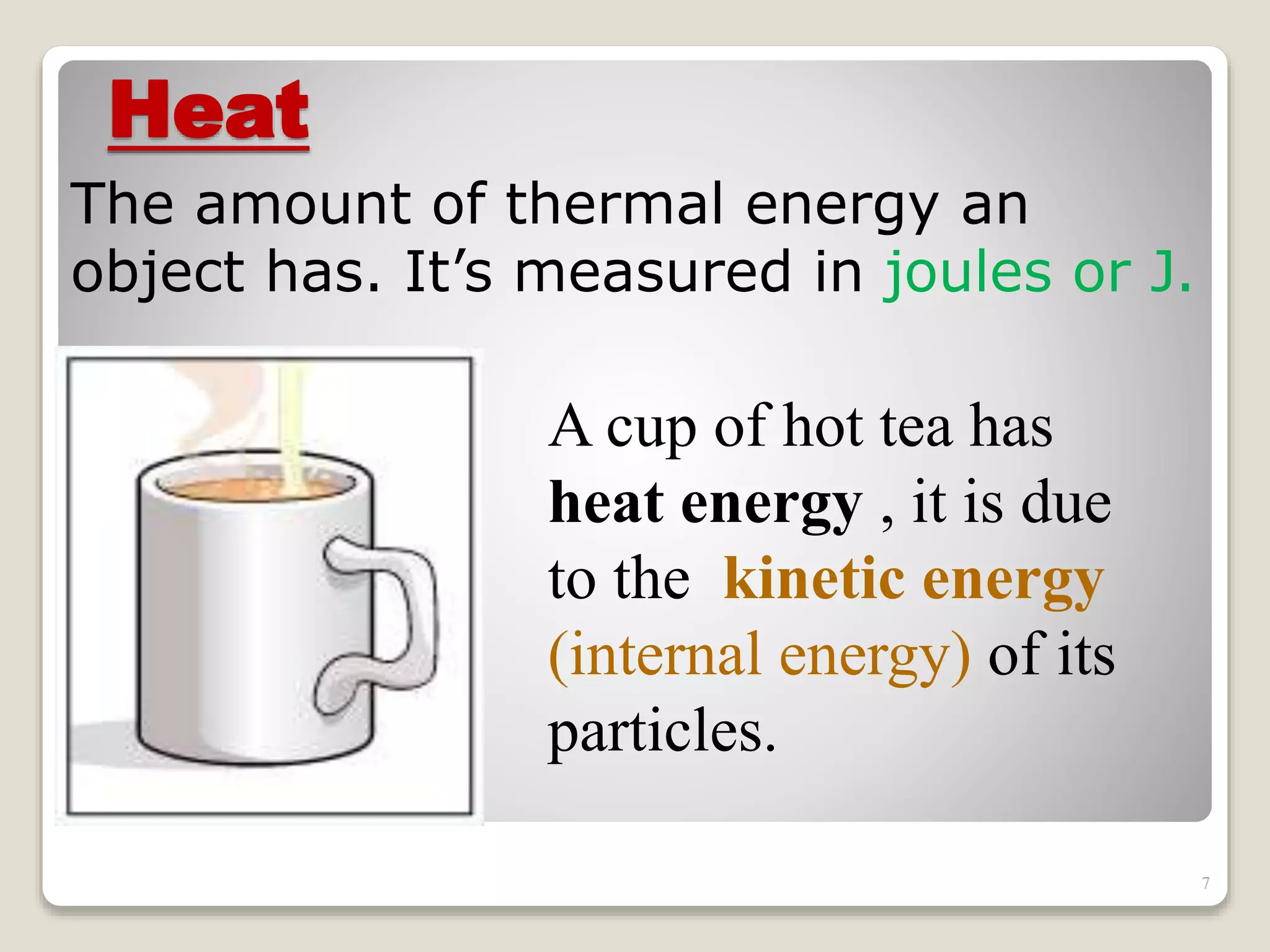
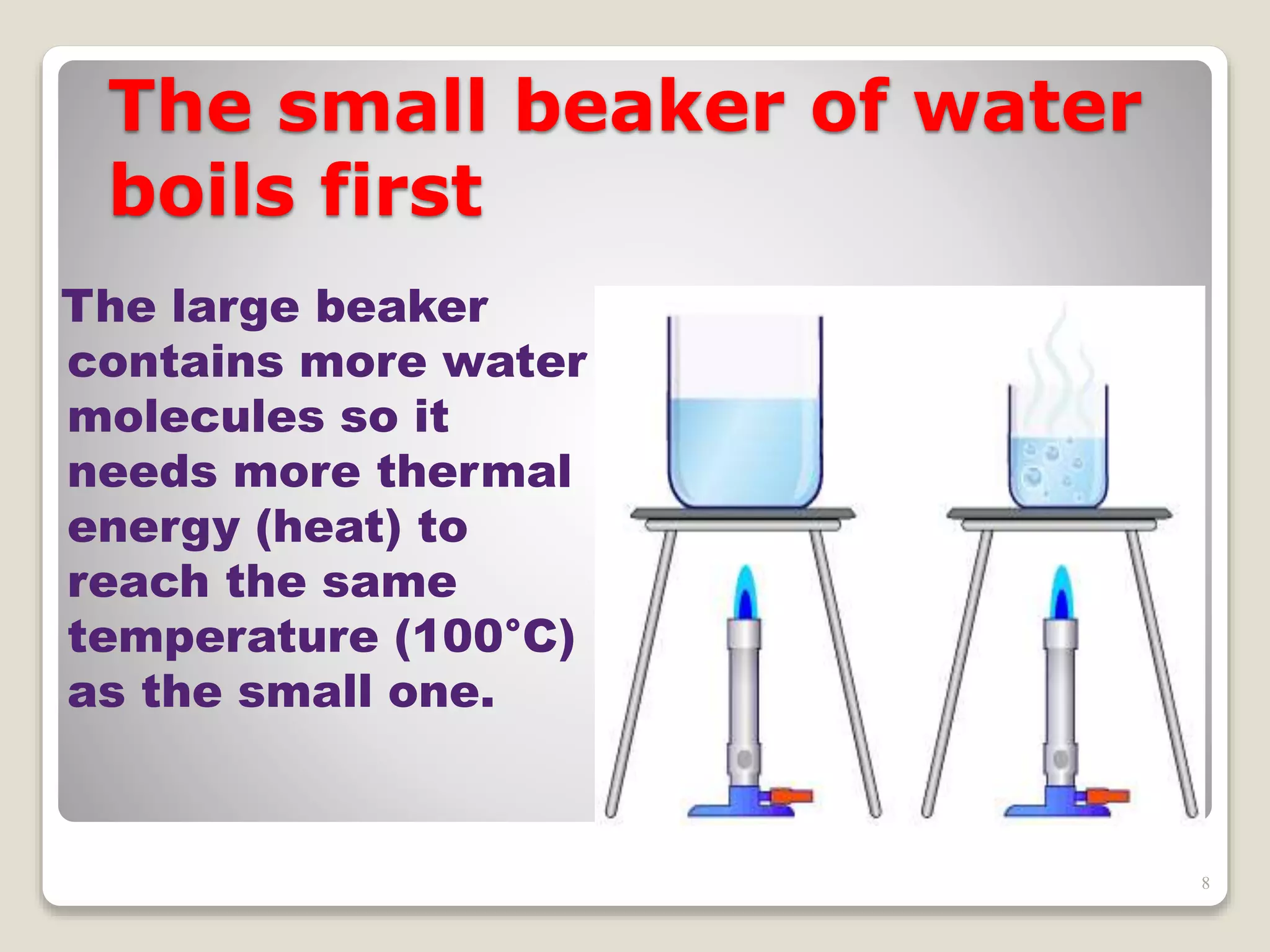
The document discusses several key concepts related to temperature and heat:
1. It defines temperature as a measure of the average kinetic energy of molecules, while heat is the total thermal energy within an object.
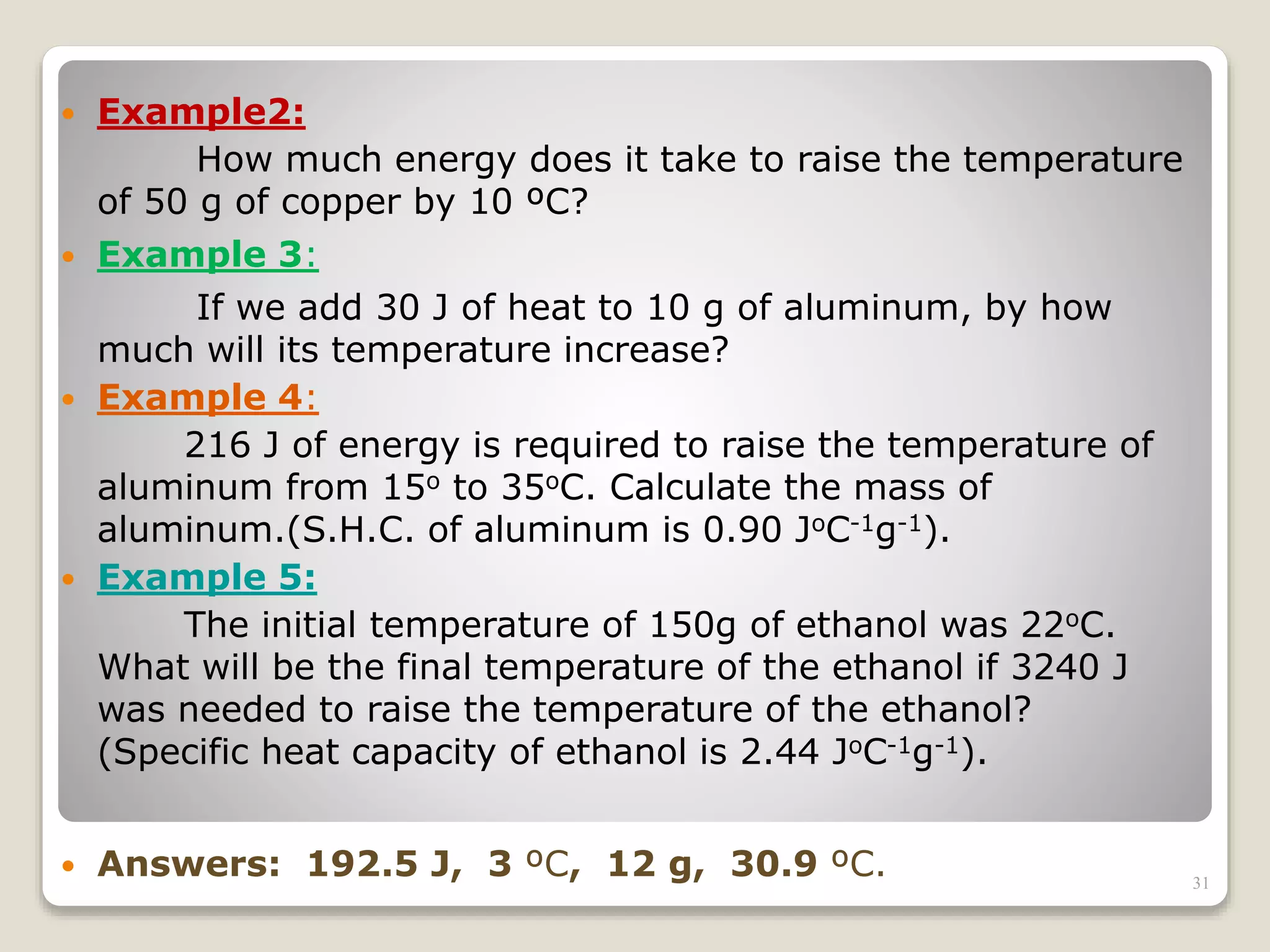
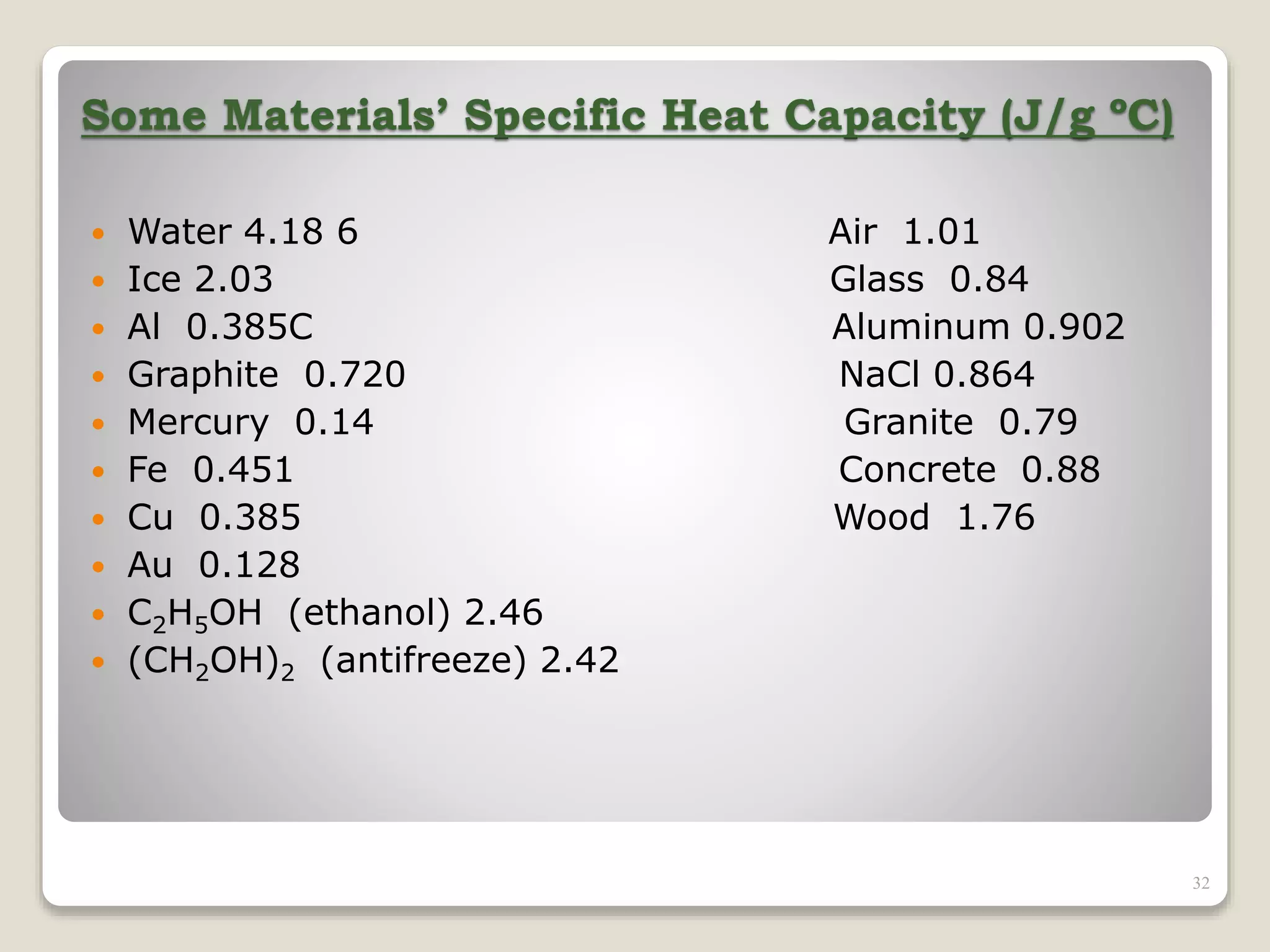
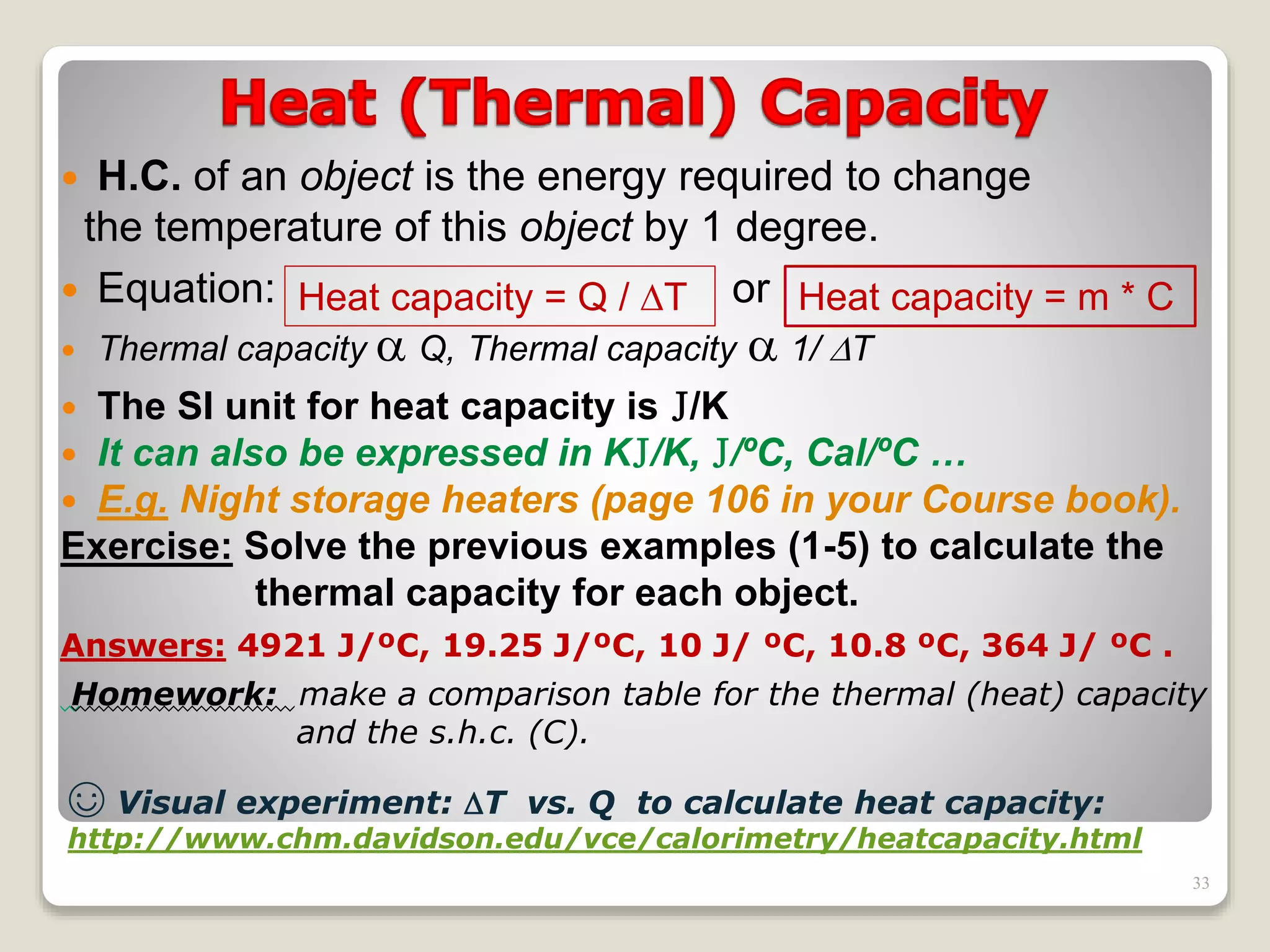
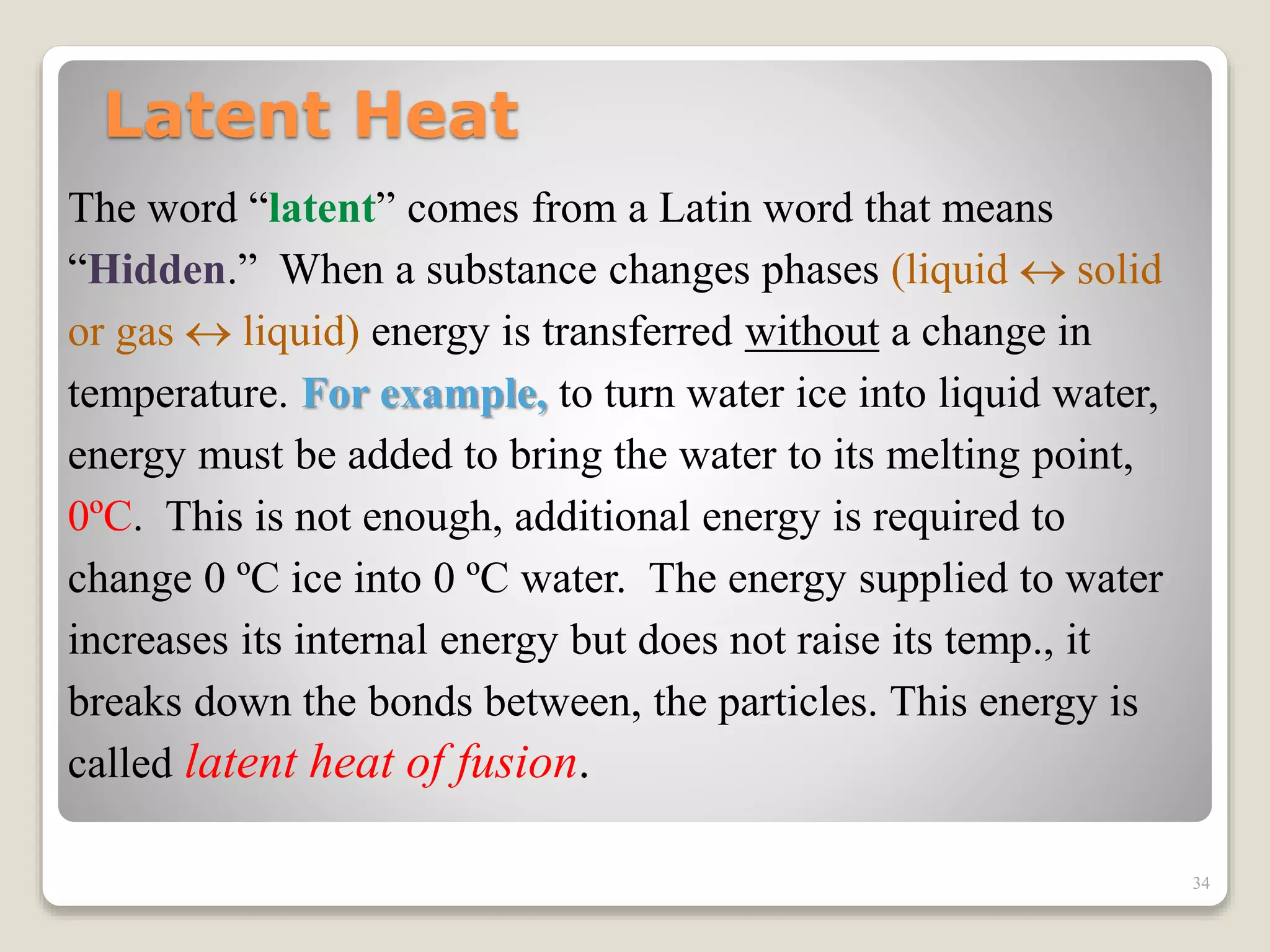
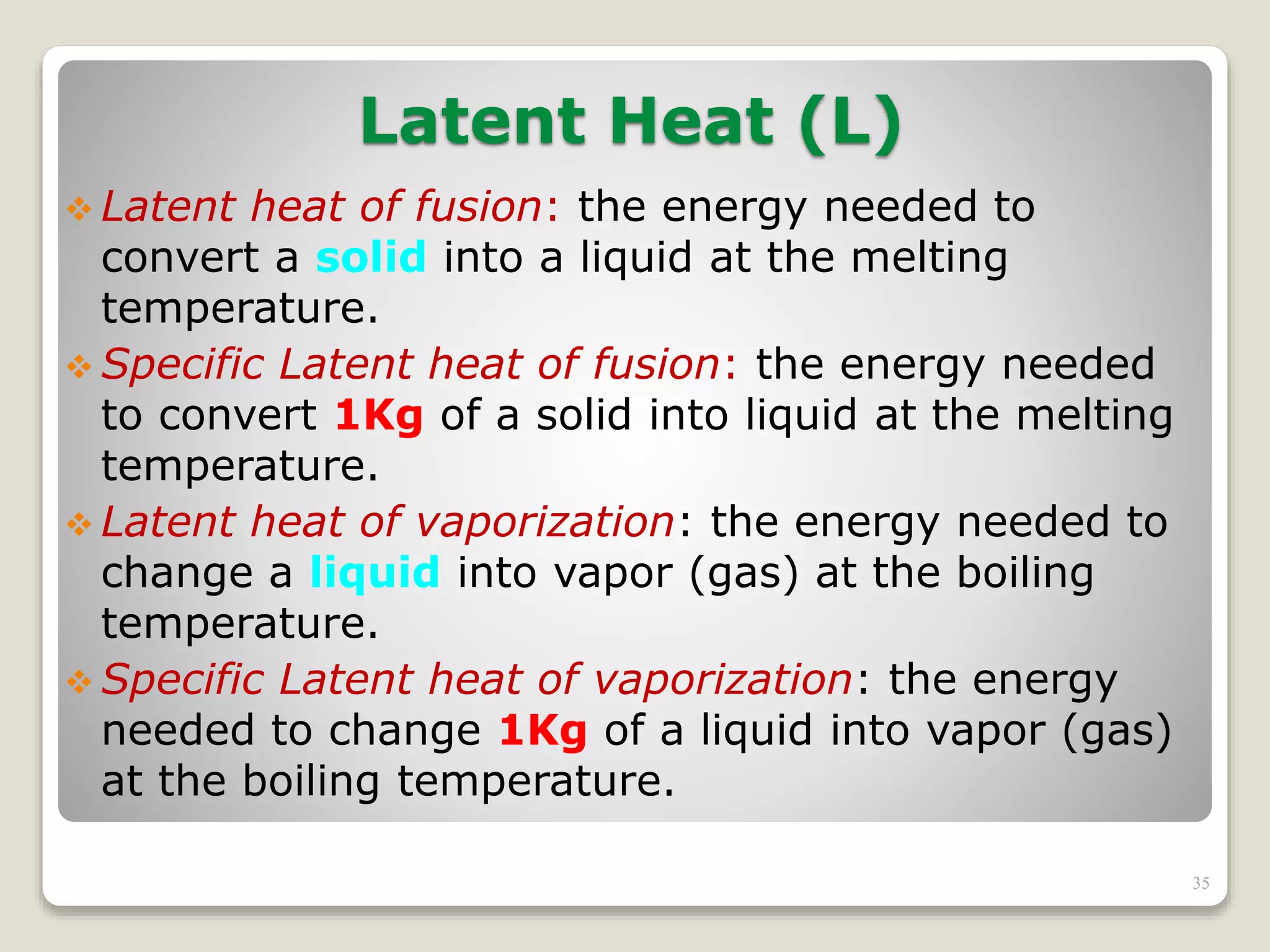
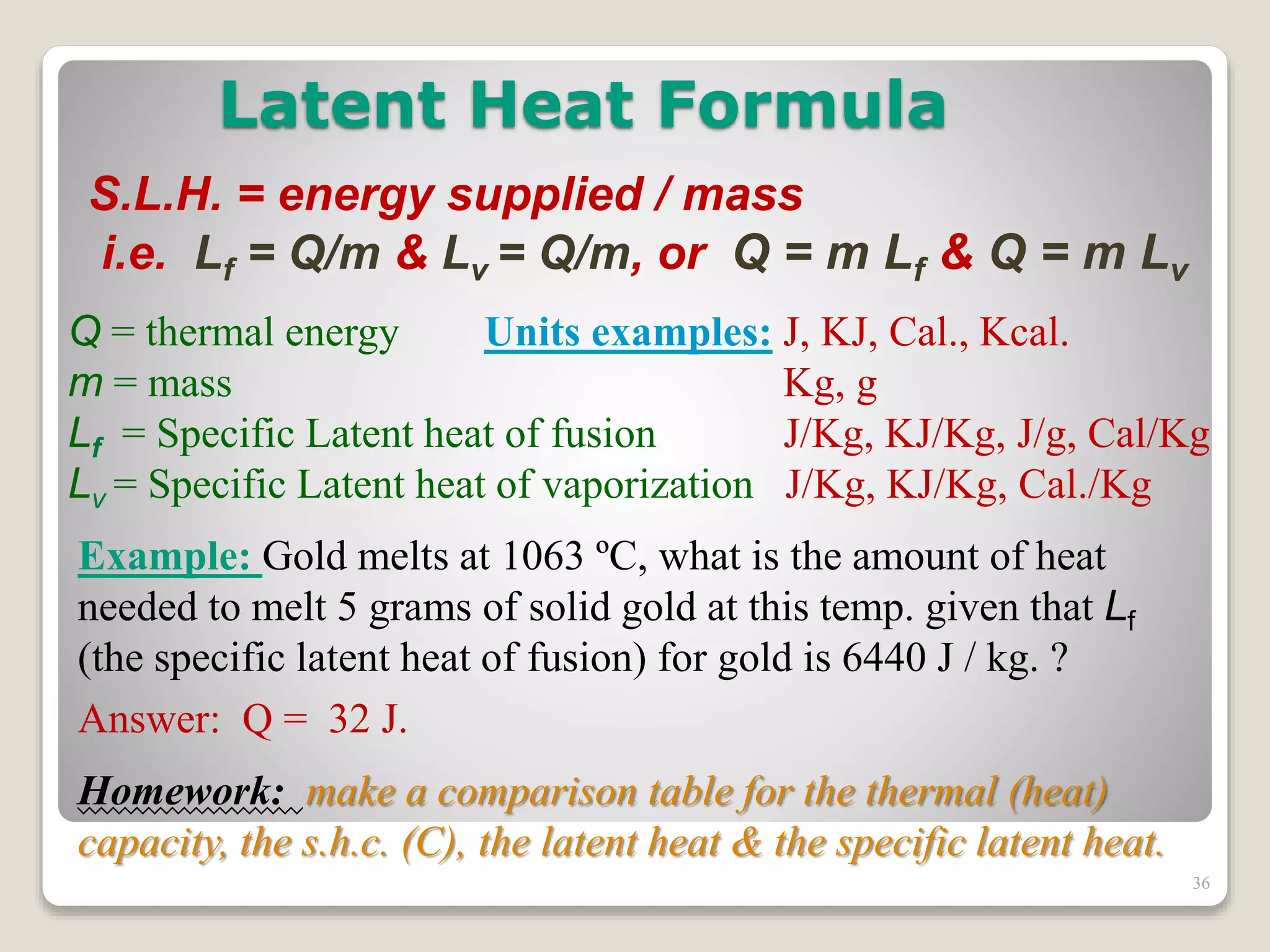
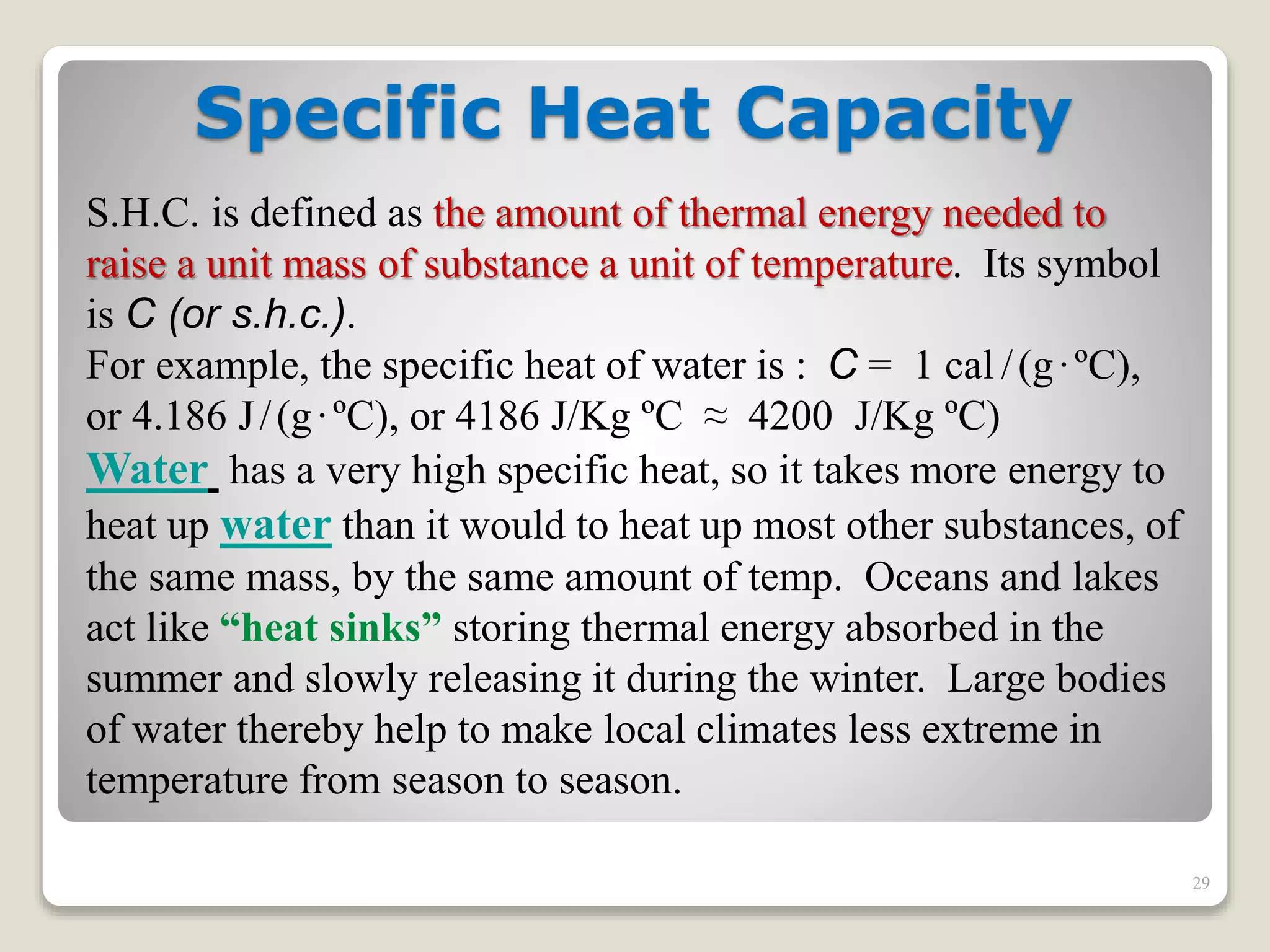
2. It explains concepts such as specific heat capacity, which is the amount of energy required to raise the temperature of a substance, and latent heat, which is the energy required for phase changes without a change in temperature.
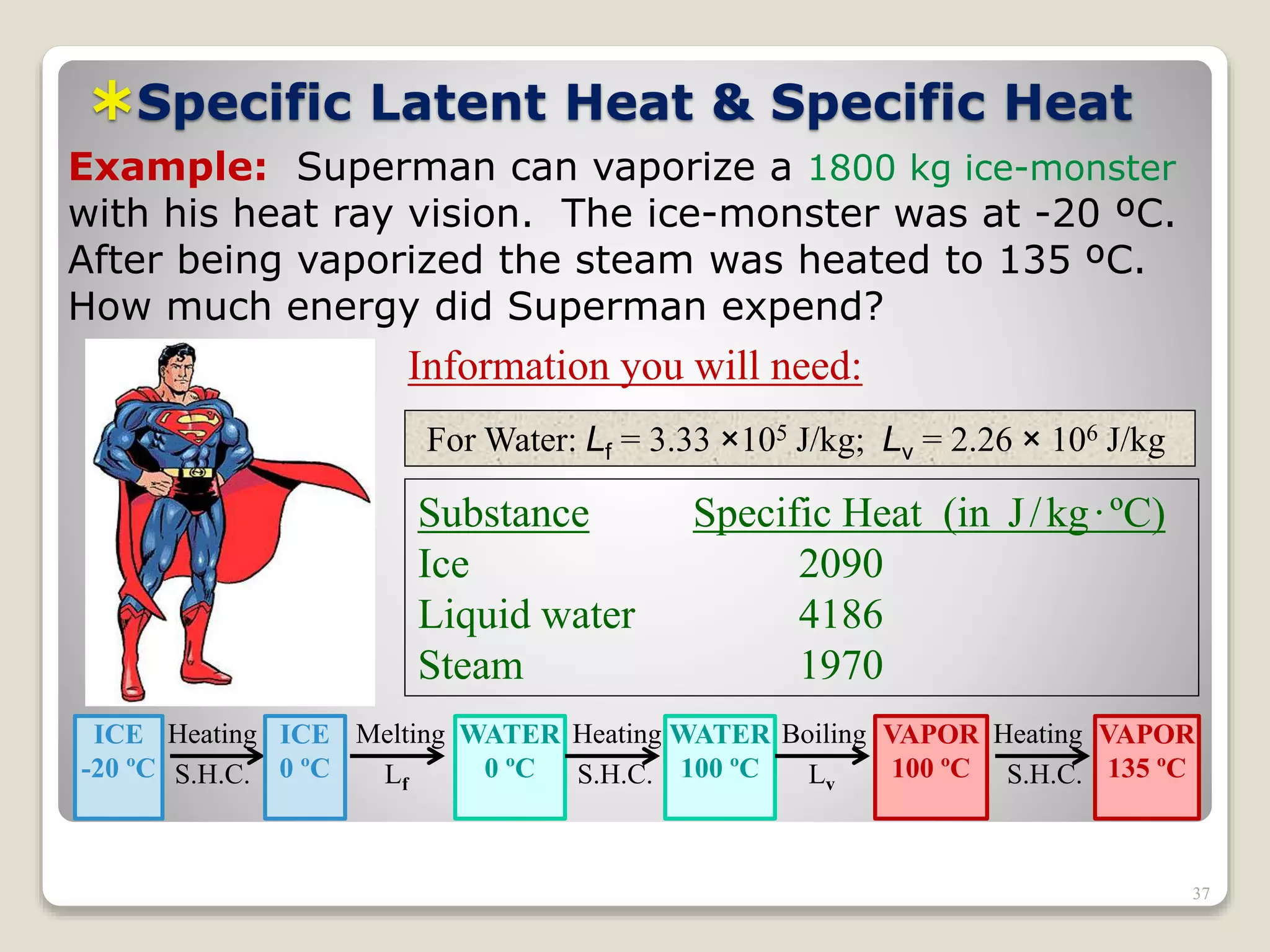
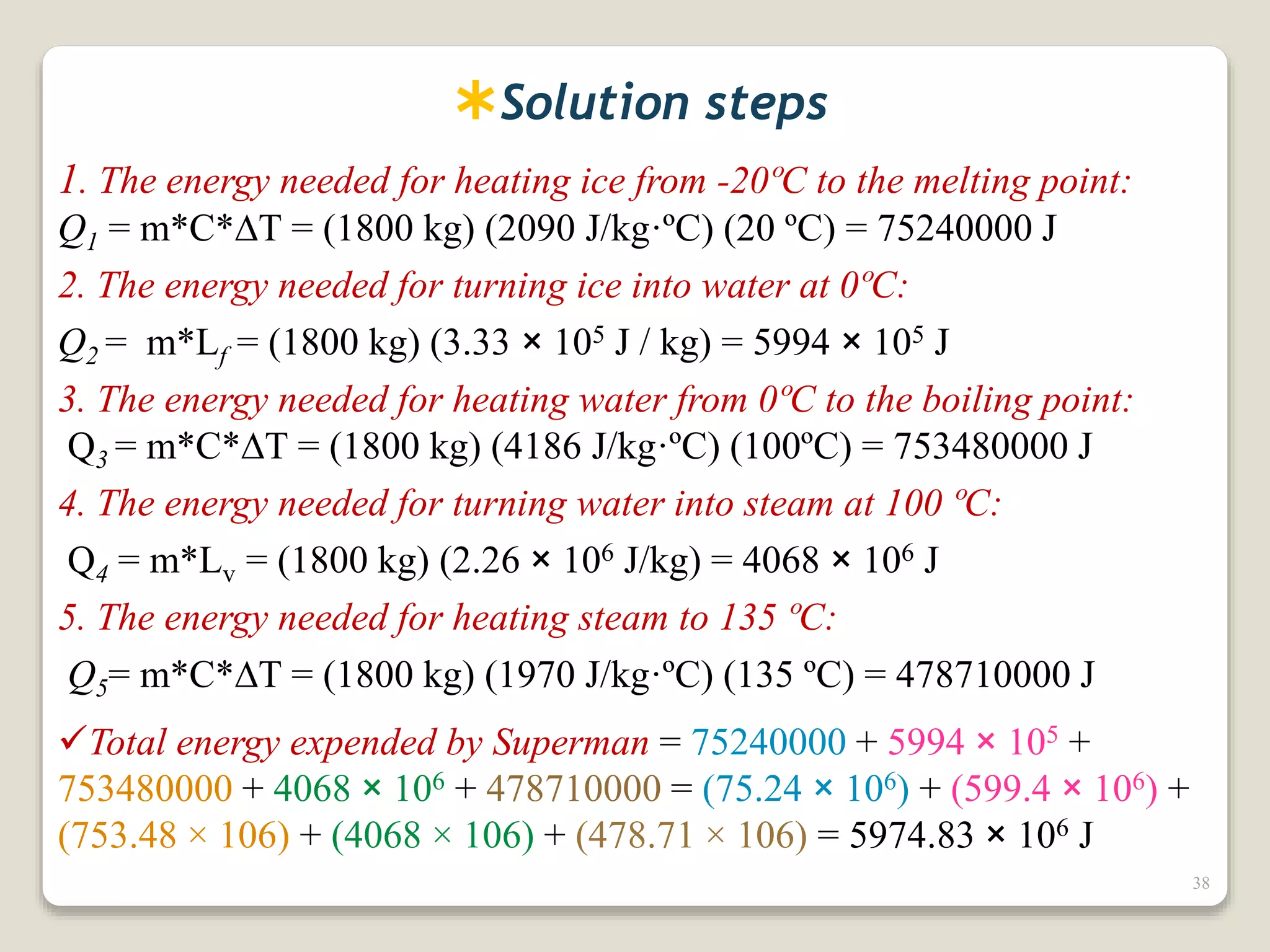
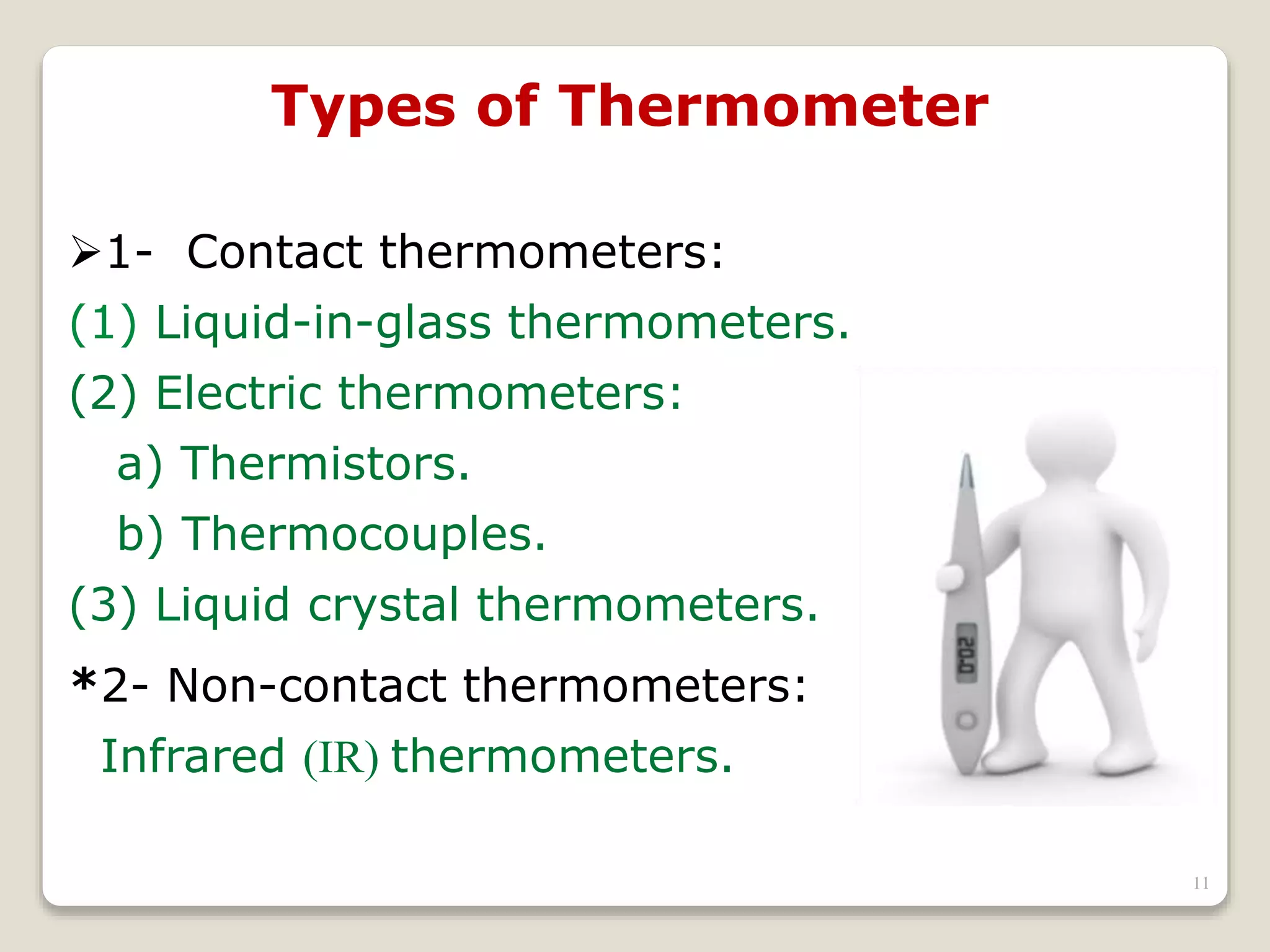
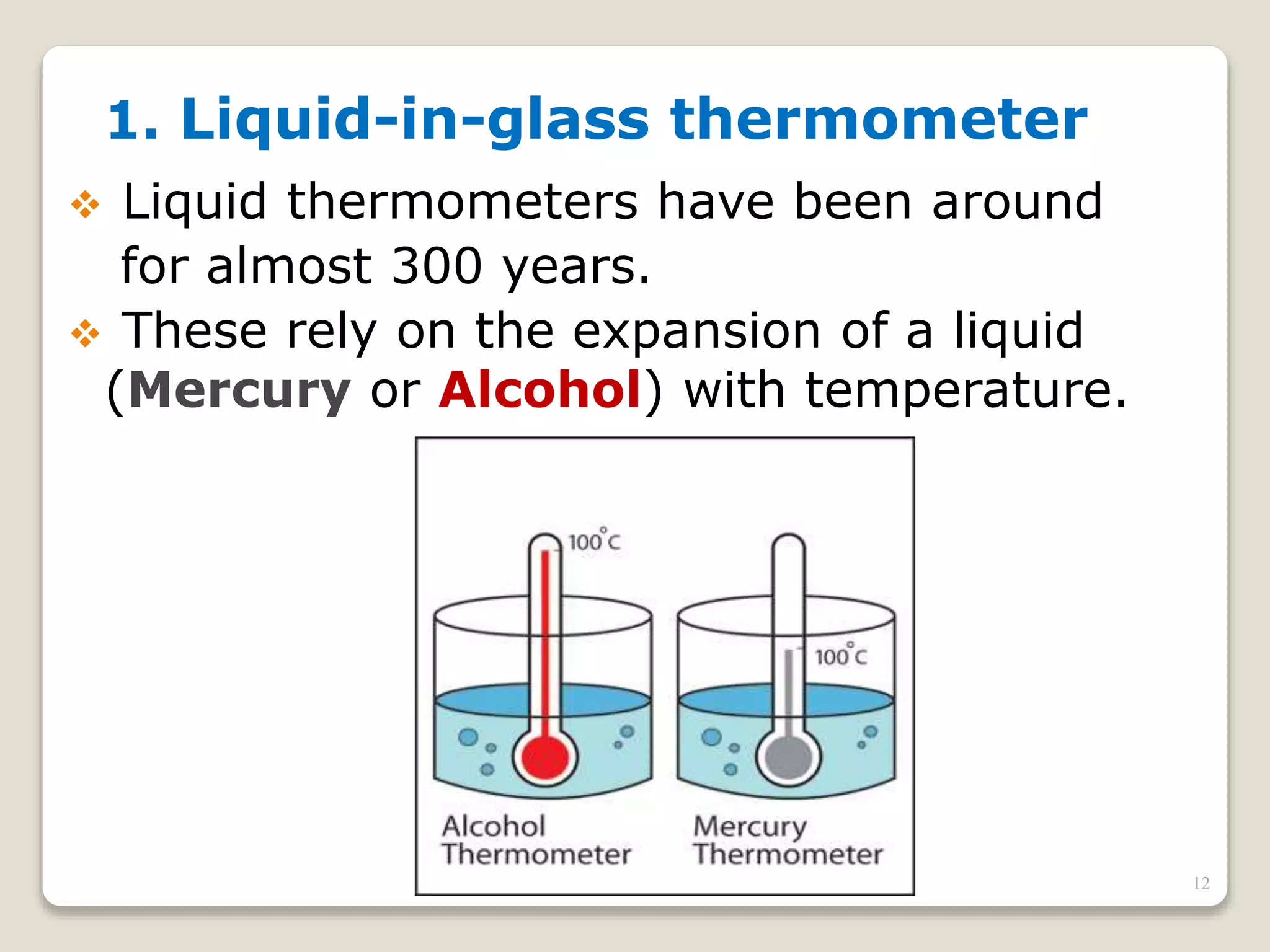
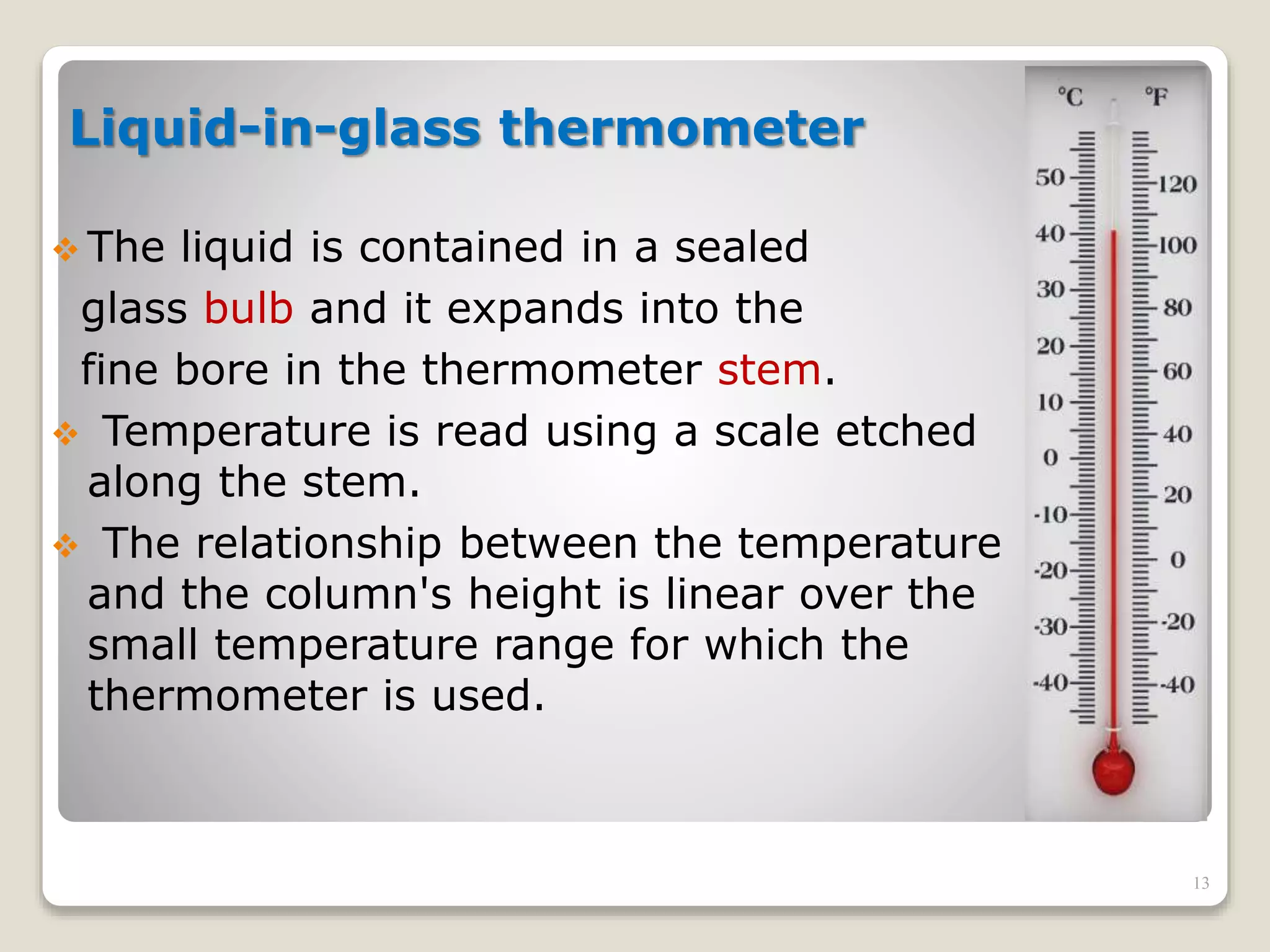
3. It discusses various types of thermometers and temperature scales, and provides examples of calculating heat transfer and temperature change using equations for specific heat, latent heat, and thermal expansion.





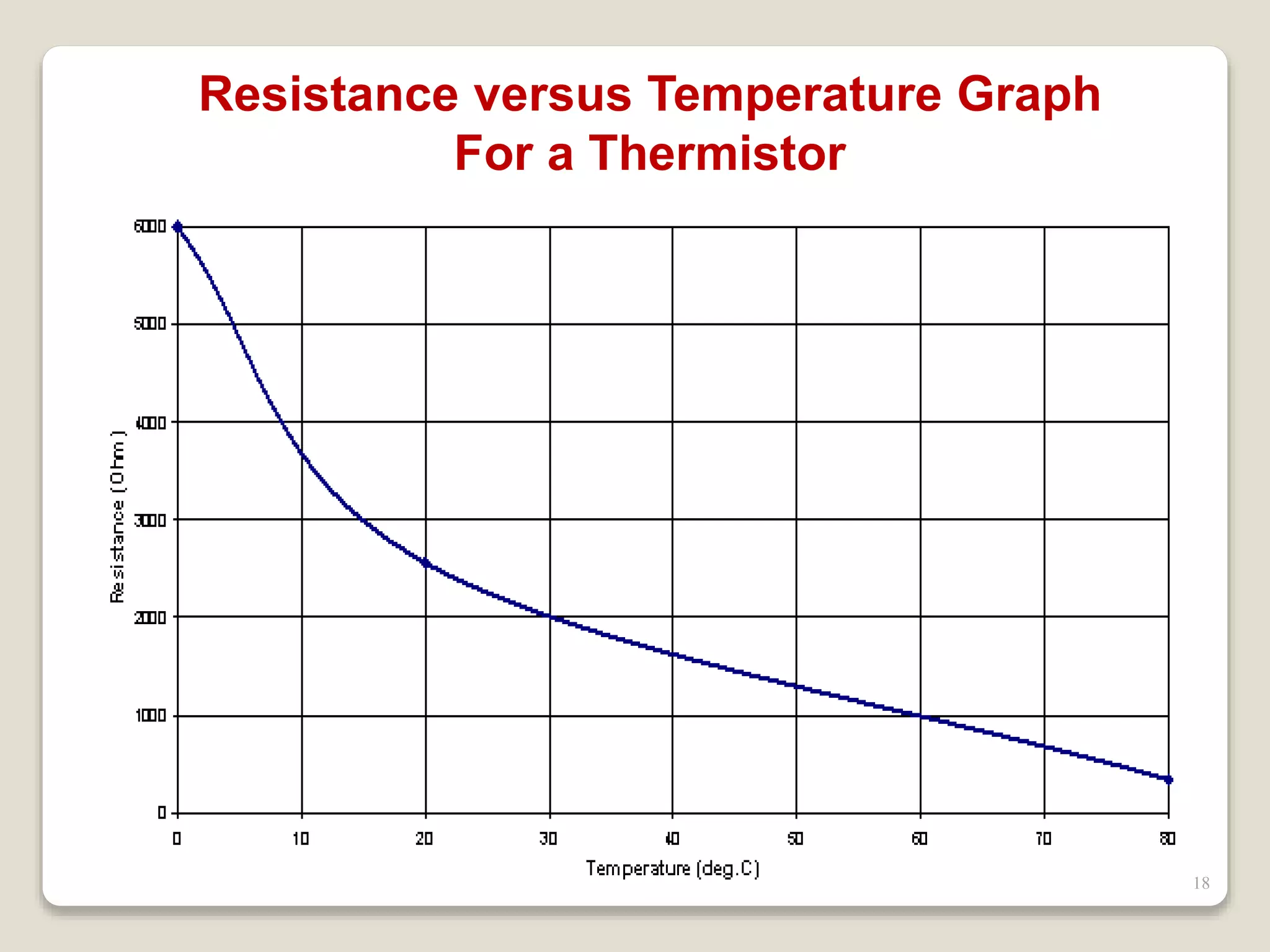

![Temperature scales
Celsius Scale:
Celsius is the metric scale for
measuring temperature.
Water freezes at 0ºC and boils at
100ºC.
Fahrenheit Scale: water freezes
at 32 °F, and boils at 212 °F
[F = 1.8C + 32]
25](https://image.slidesharecdn.com/capter10-140508160606-phpapp01/75/Capter-10-for-9th-grade-Physics-25-2048.jpg)


![Specific Heat Equation
Q = m C T
Q = thermal energy (J, or KJ, or cal, or Cal …)
m = mass (g, or Kg …)
T = change in temp. (ºC, or ºF, or Kelvin)
C = specific heat capacity
Example1: The specific heat of silicon is 703 J / (kg · ºC).
How much energy is needed to raise the temperature
of a 7 kg chunk of silicon by 10ºC ?
Solution:
703 J
kg · ºCQ = 7 kg * [ ]*10 ºC = 49 210 J
☺ Visual experiments: Measure Specific Heat Capacity of Ethanol:
http://www.chm.davidson.edu/vce/calorimetry/SpecificHeatCapacityofEthanol.html
30](https://image.slidesharecdn.com/capter10-140508160606-phpapp01/75/Capter-10-for-9th-grade-Physics-30-2048.jpg)