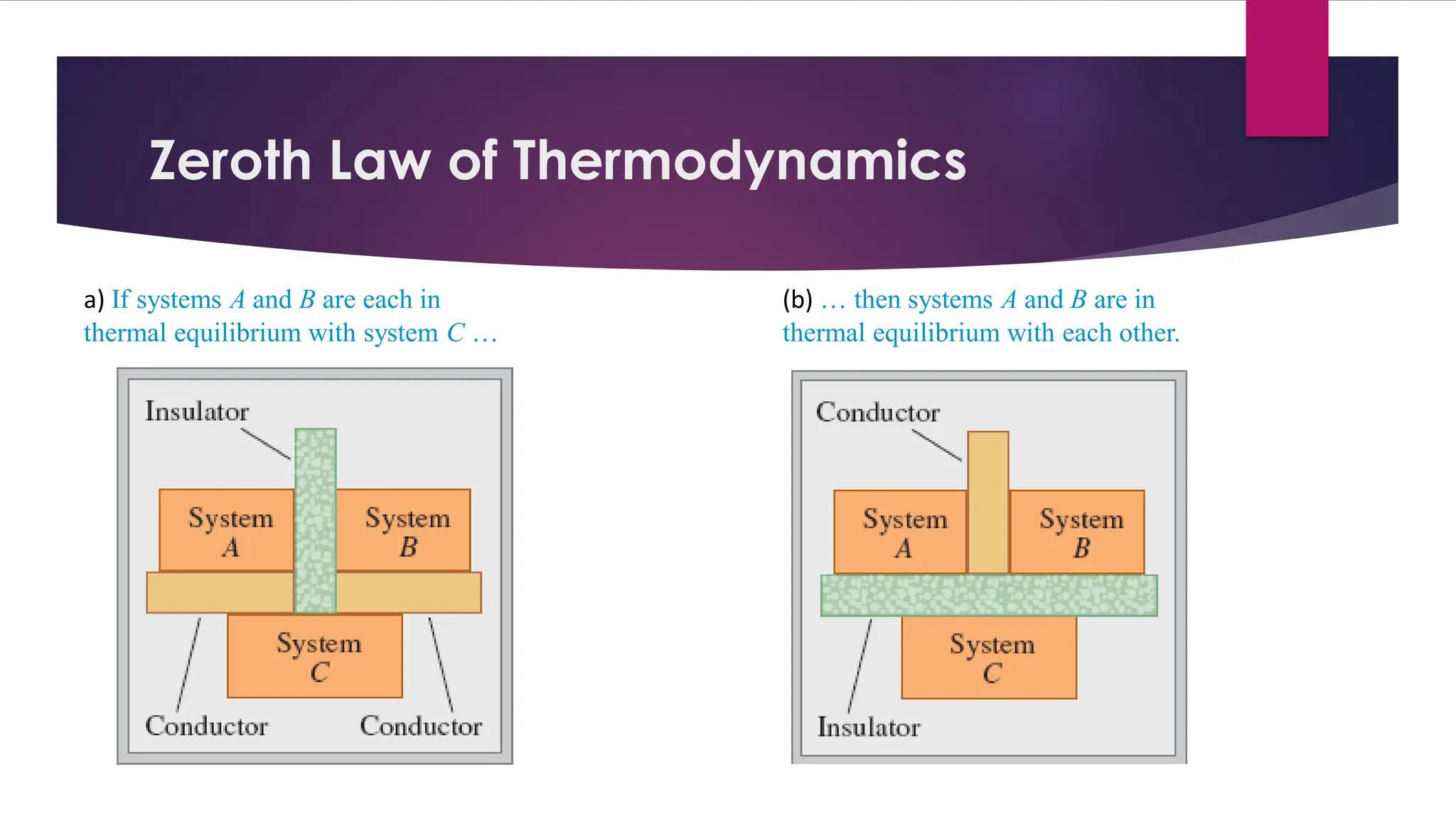
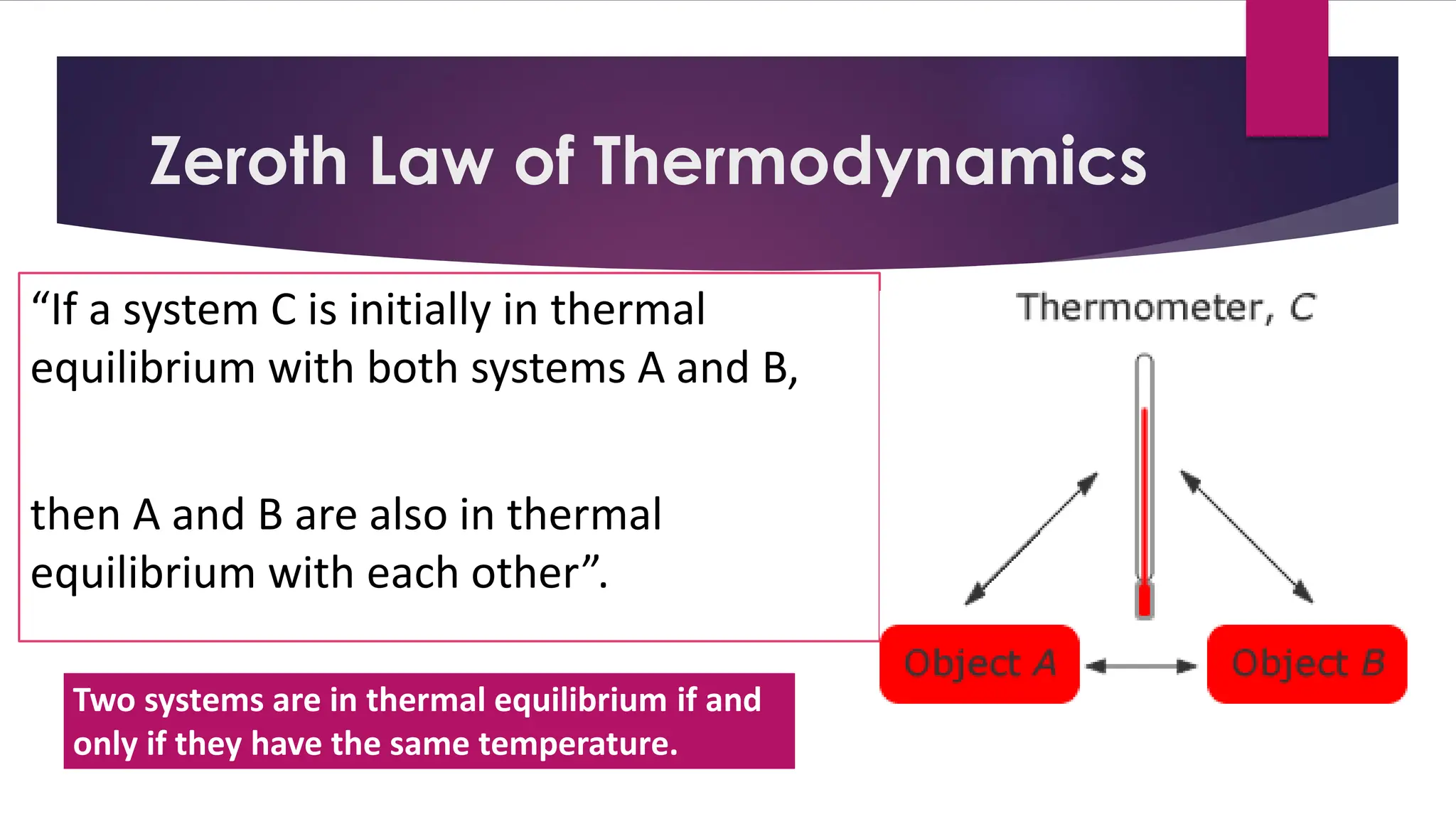
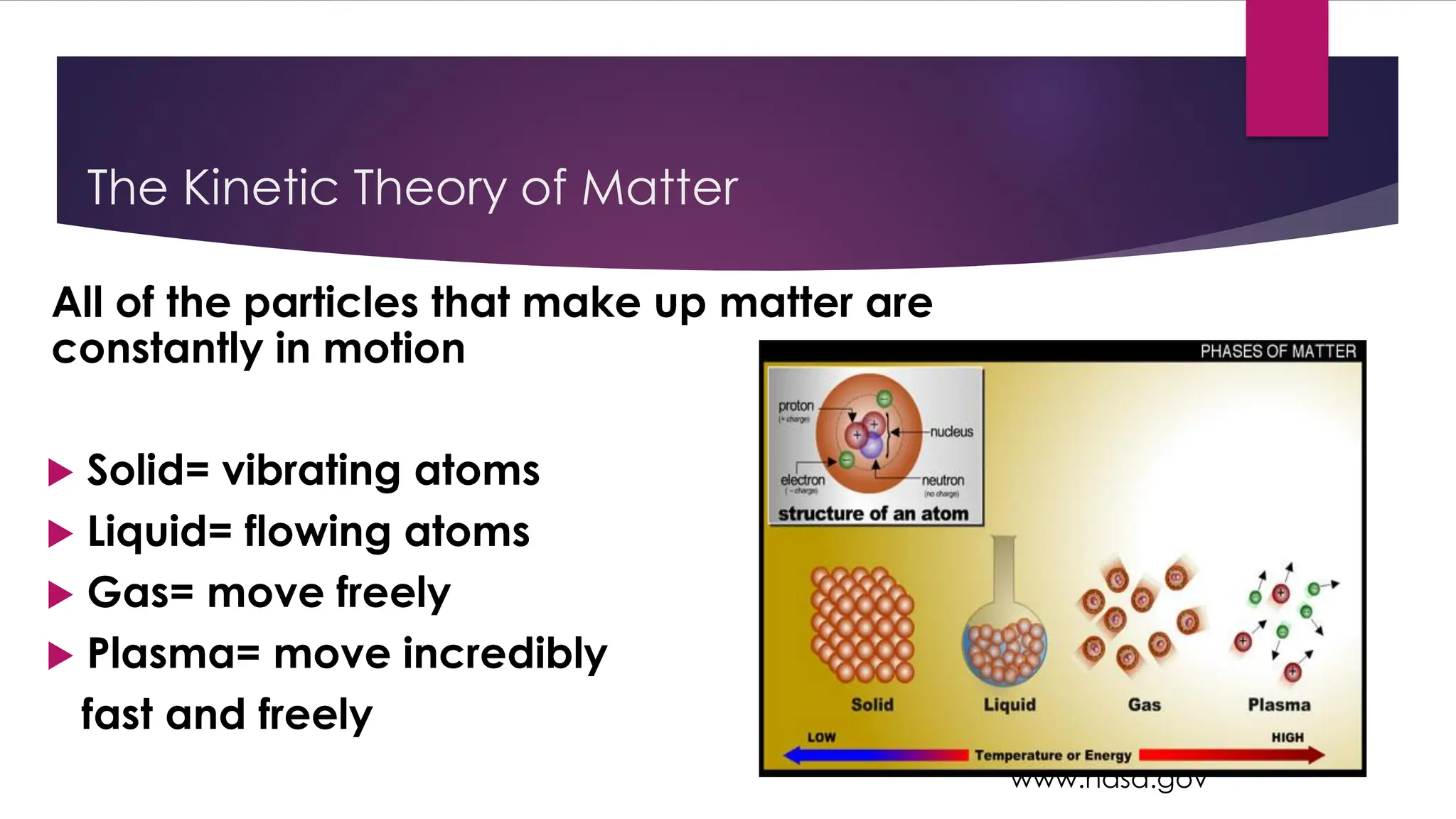
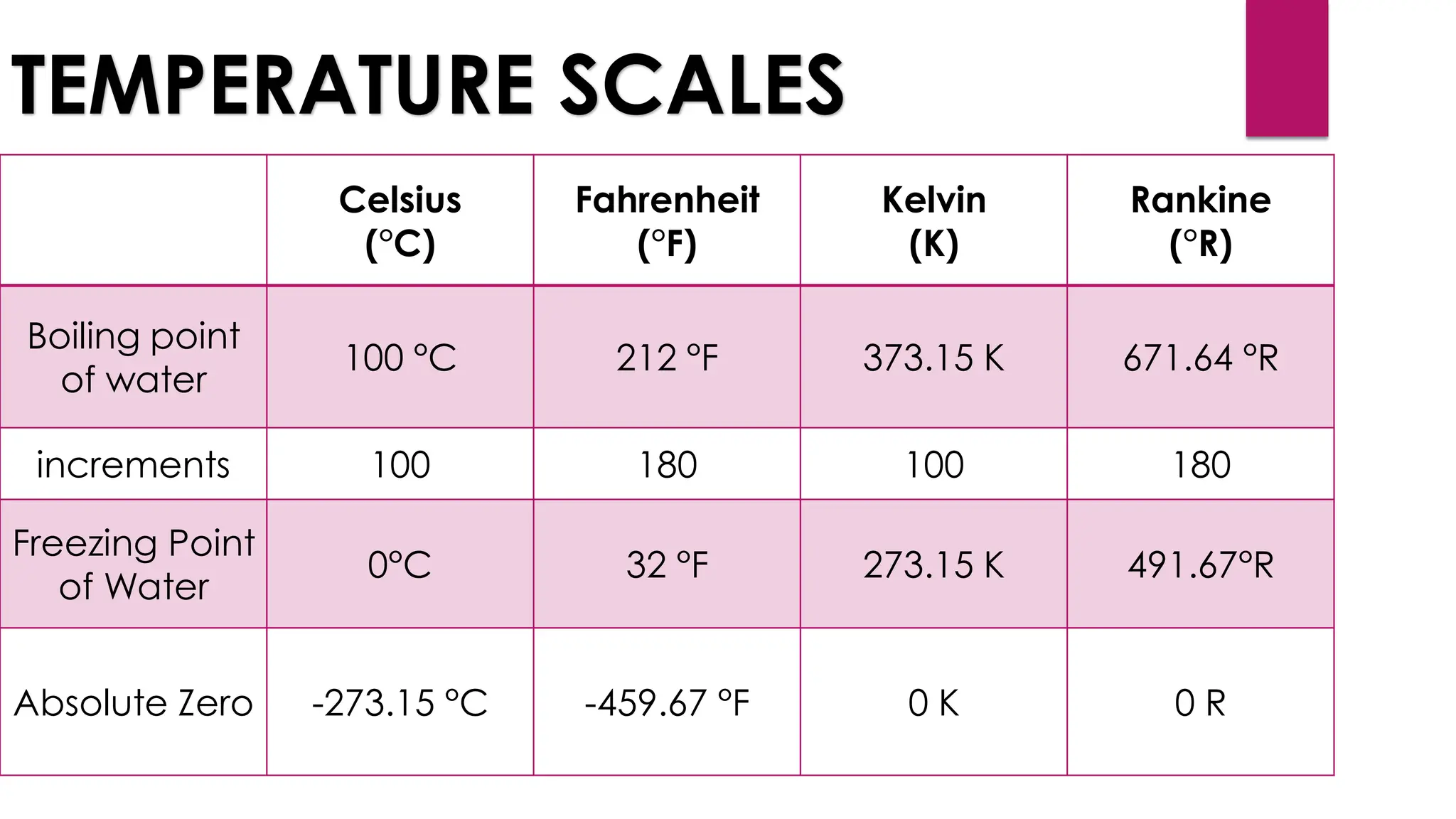
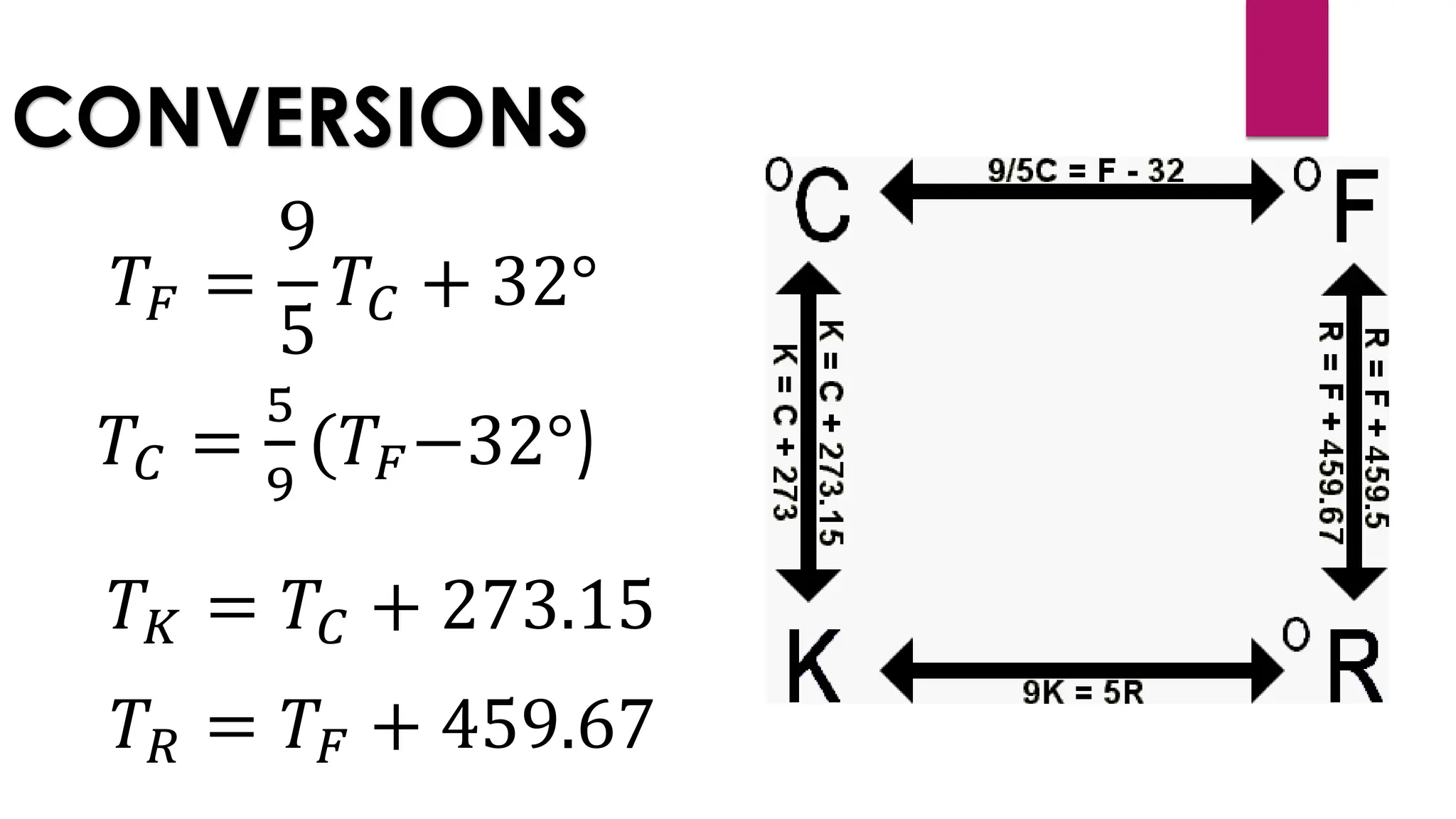
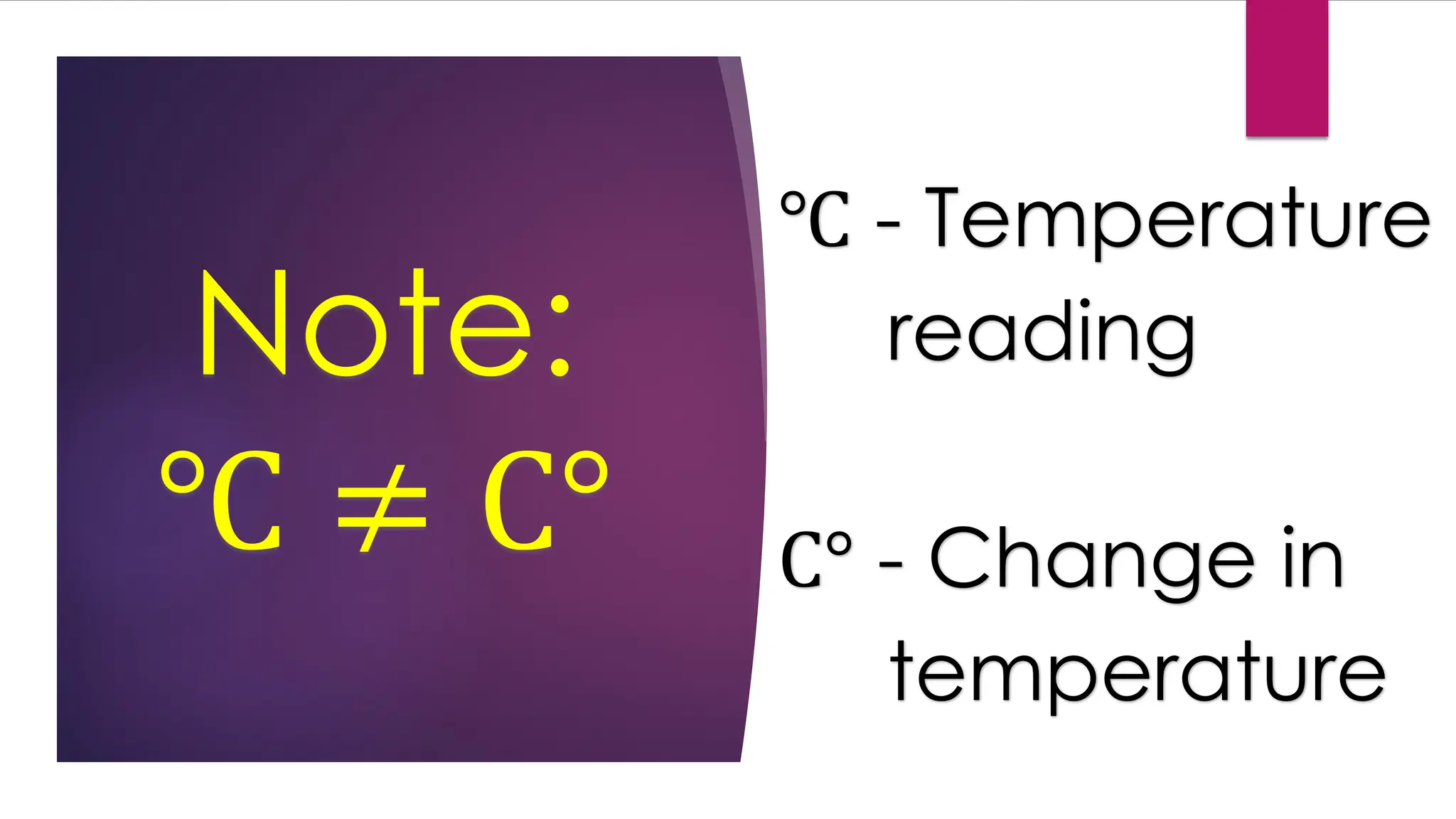
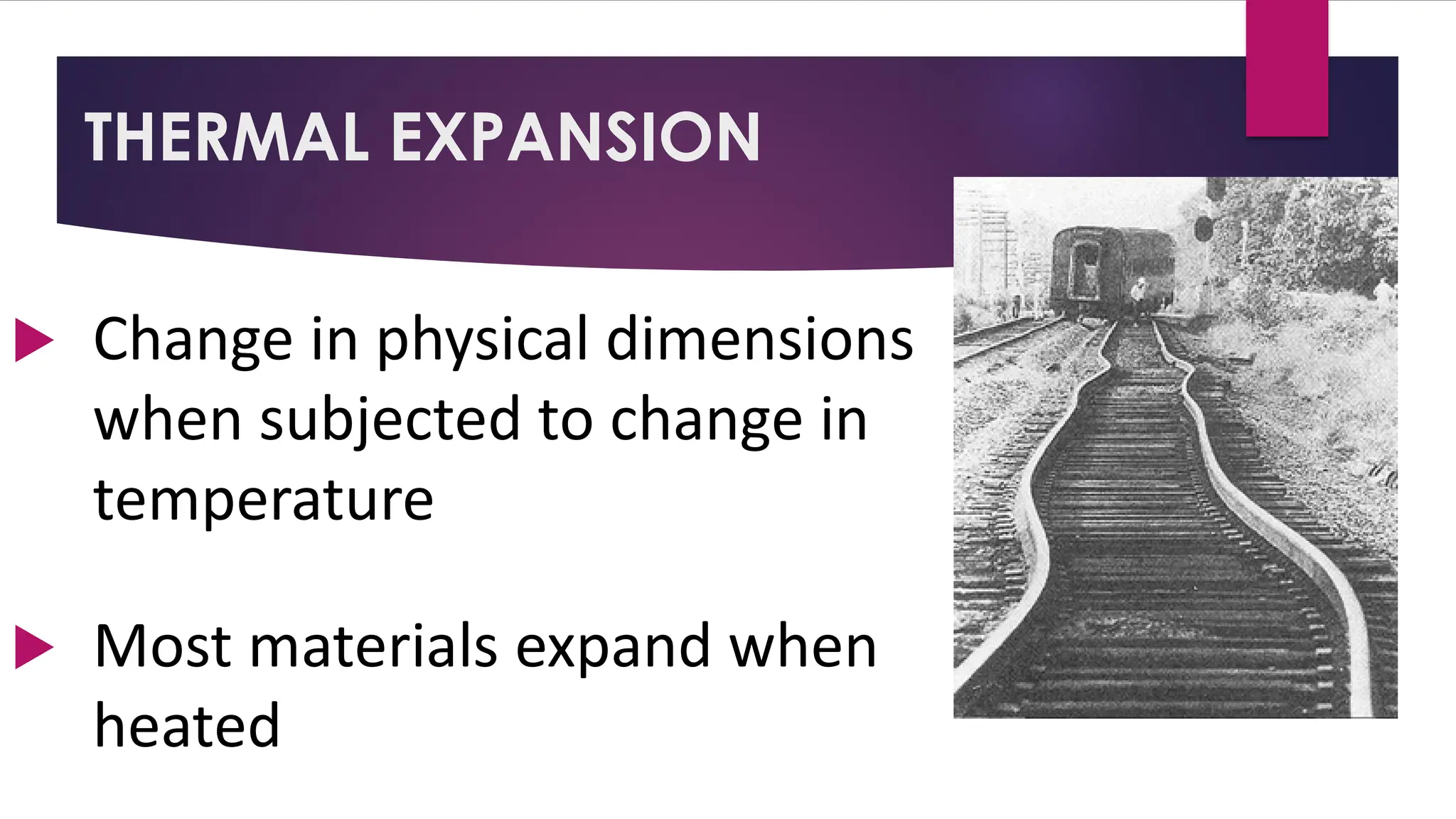
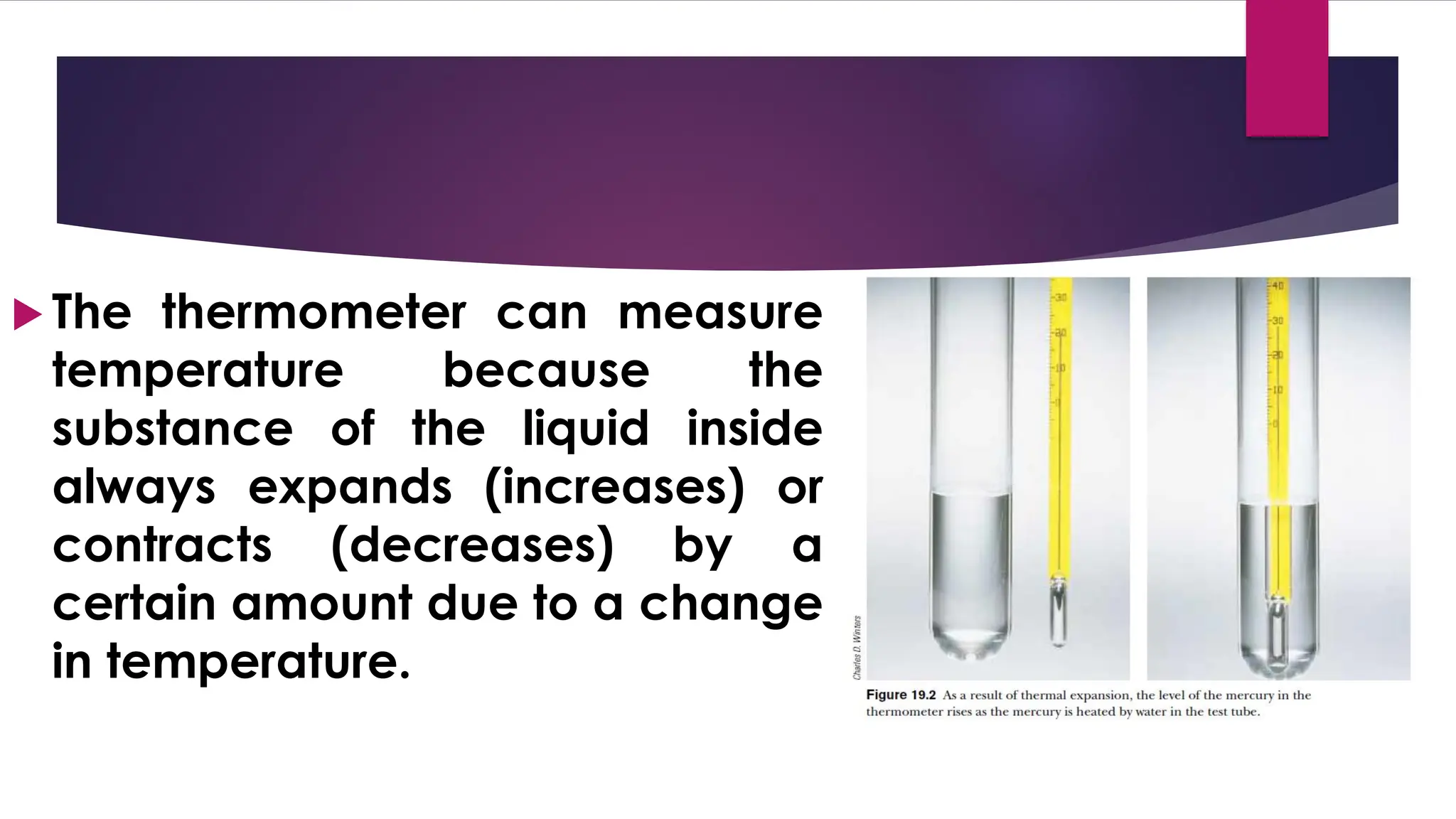
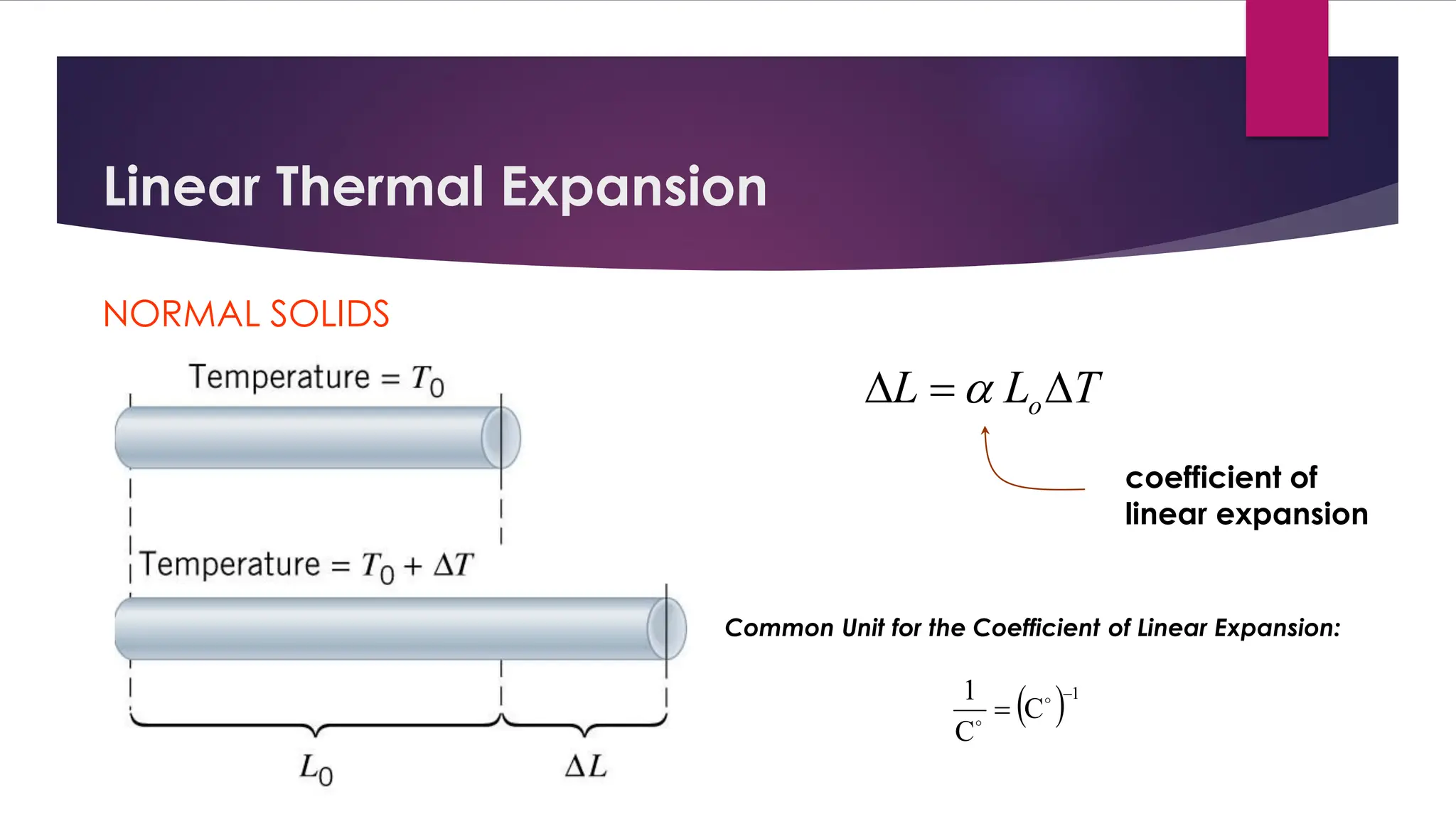
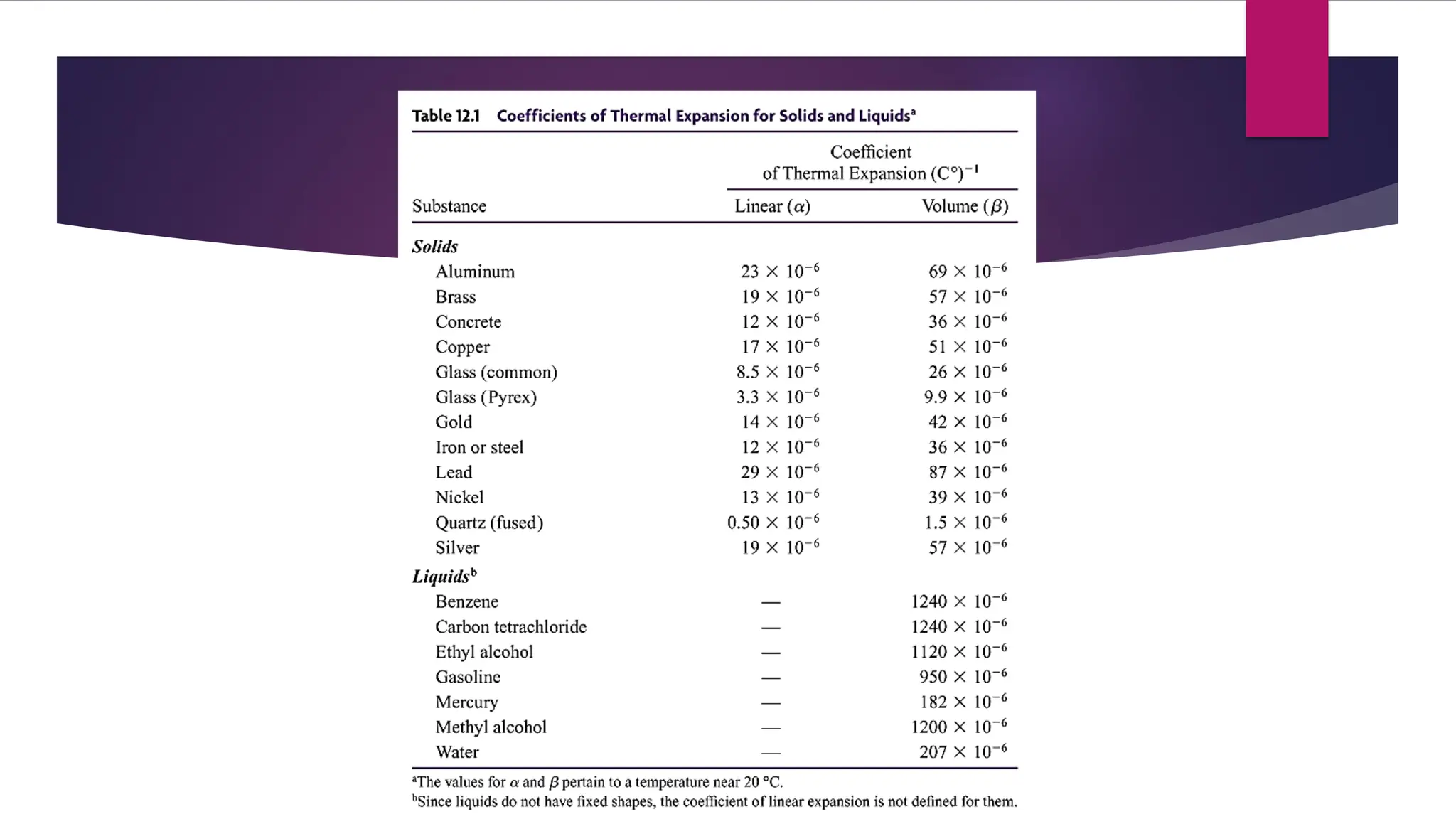
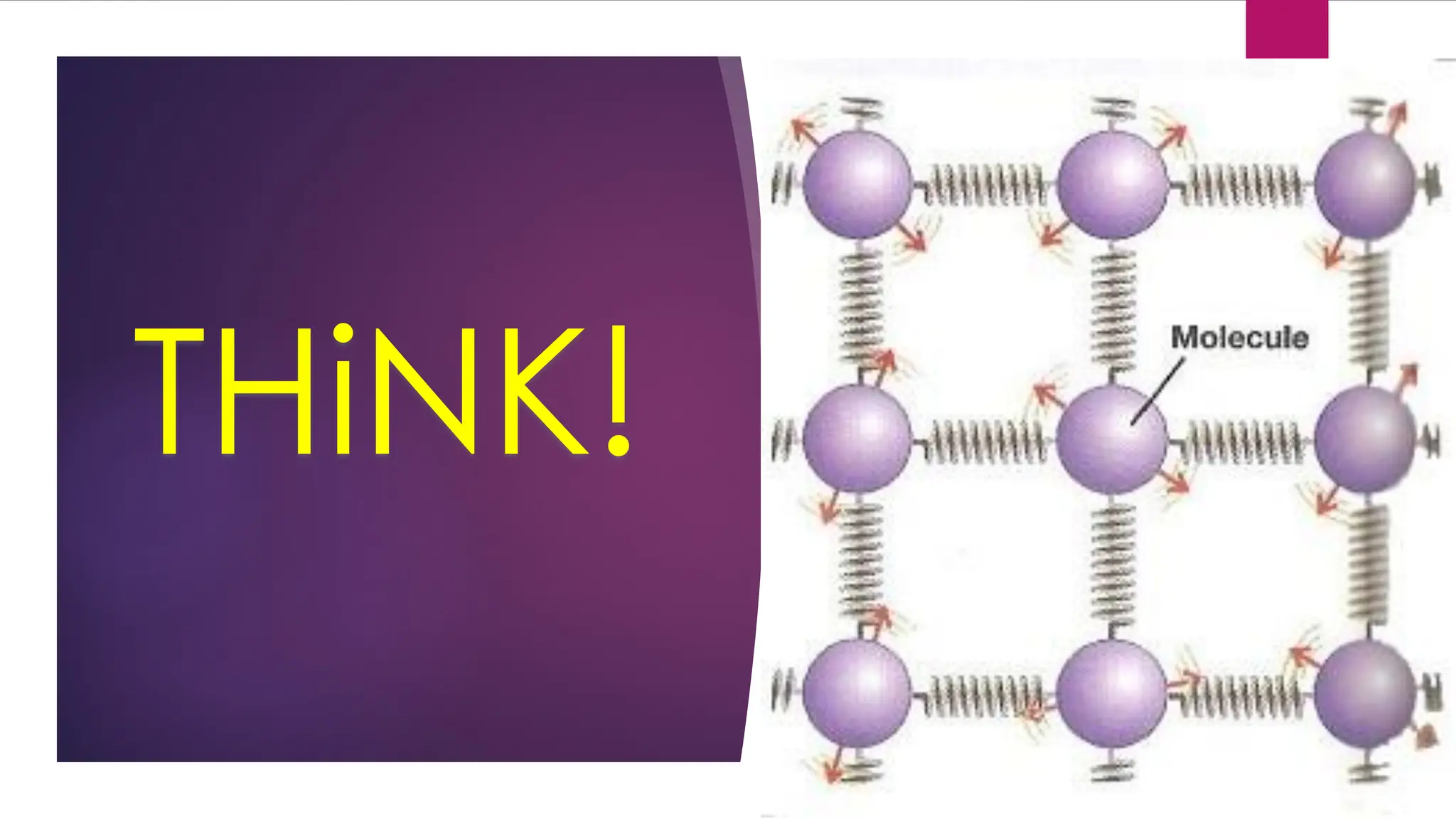
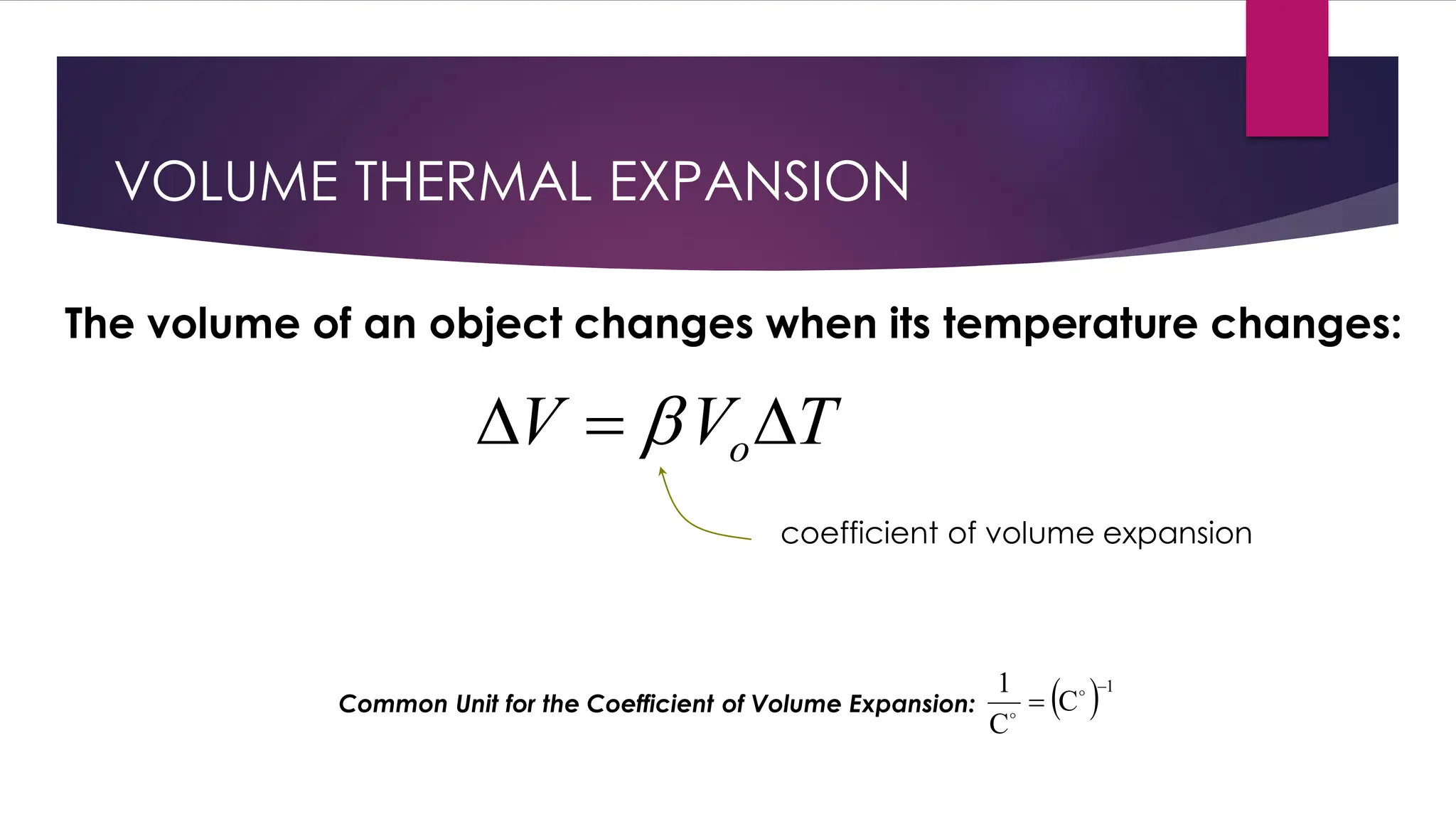
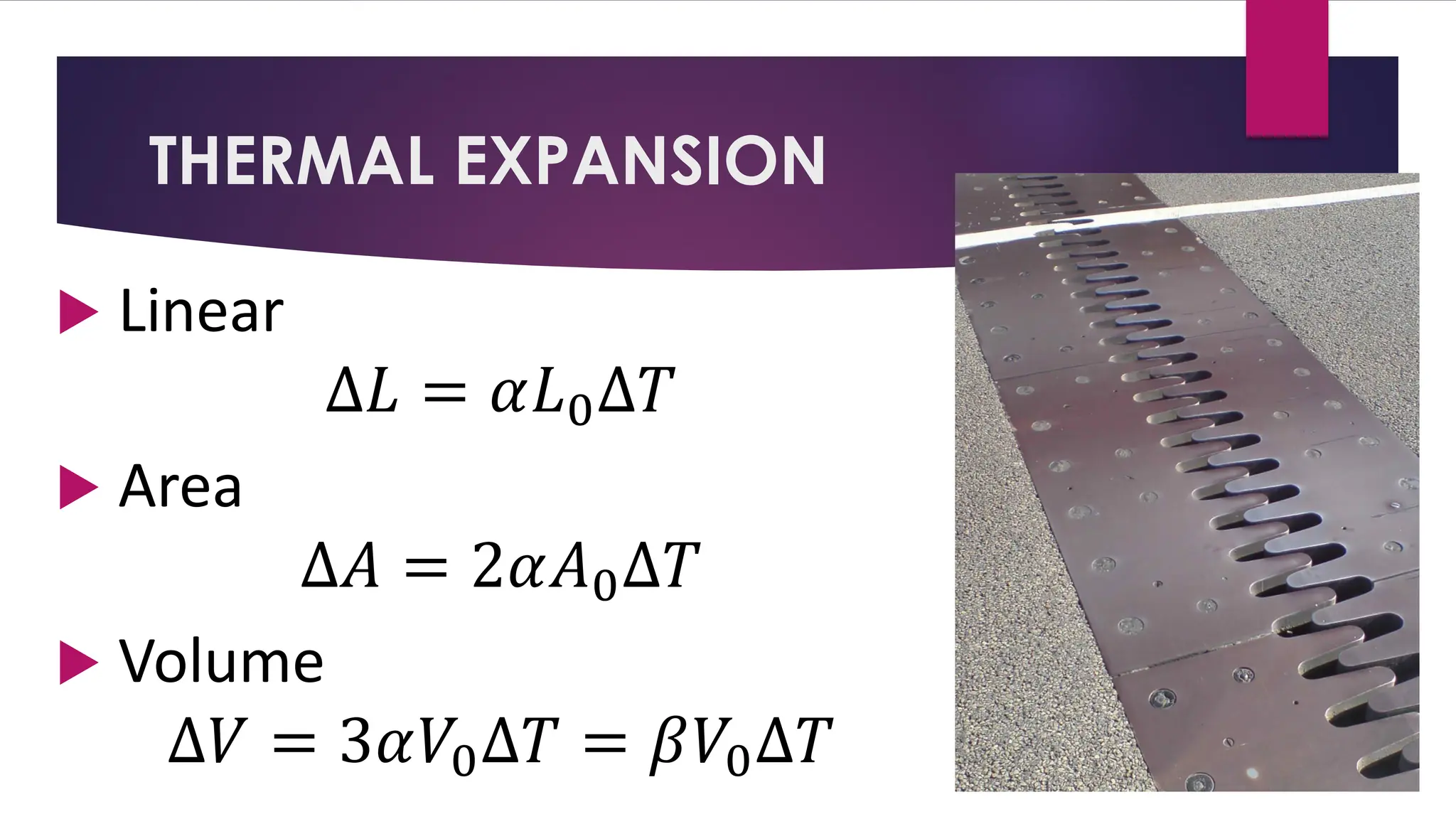
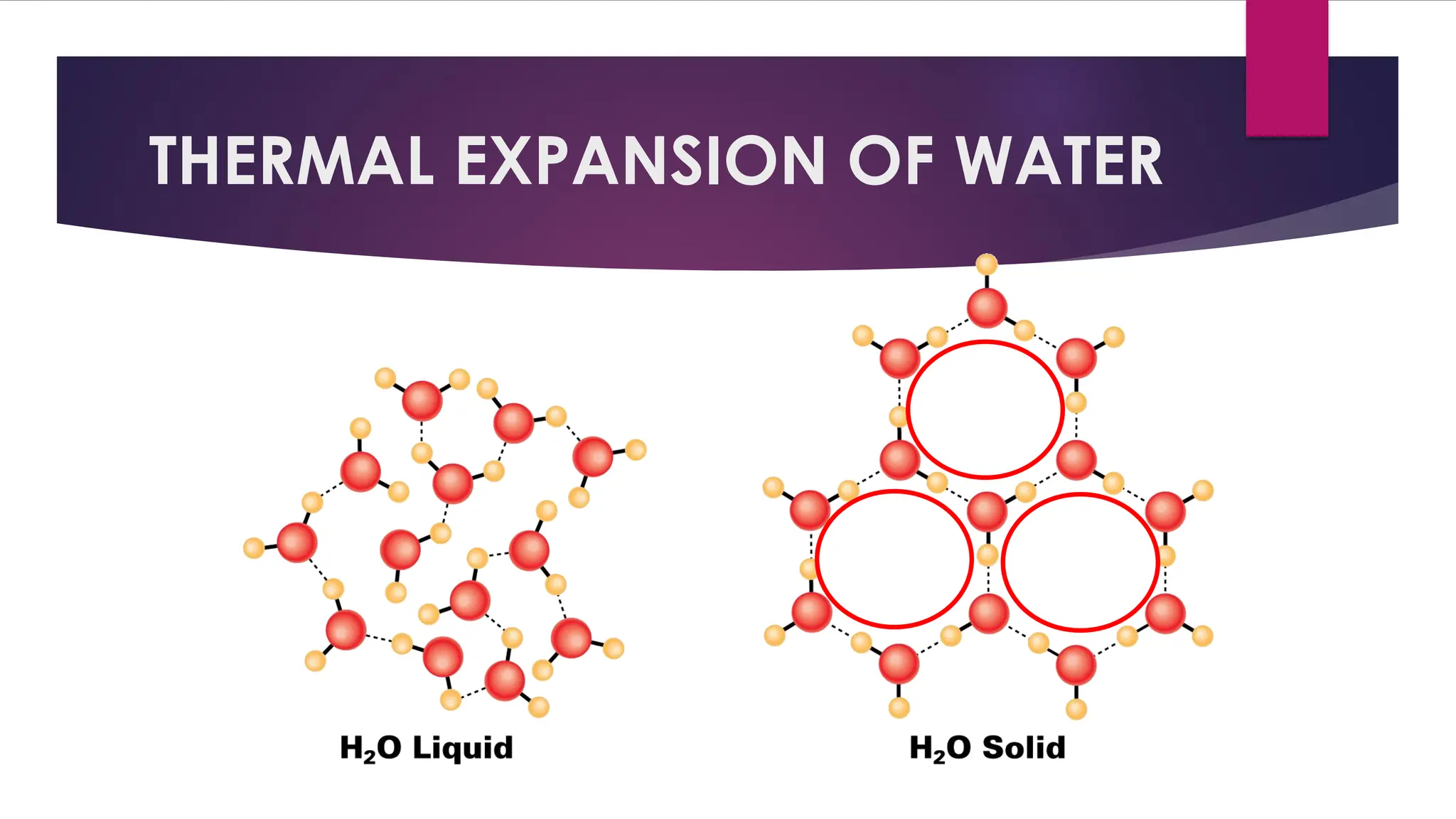
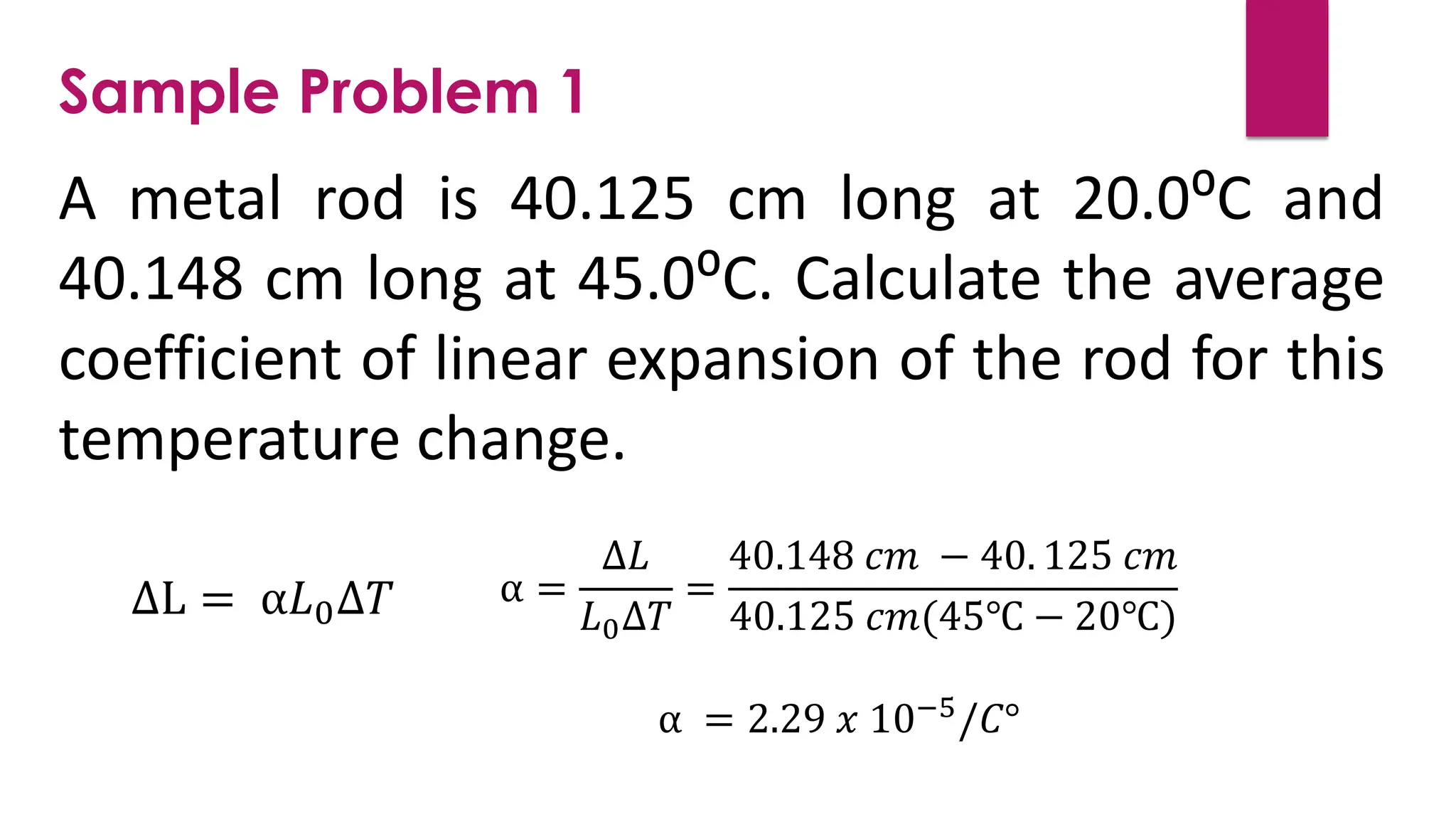
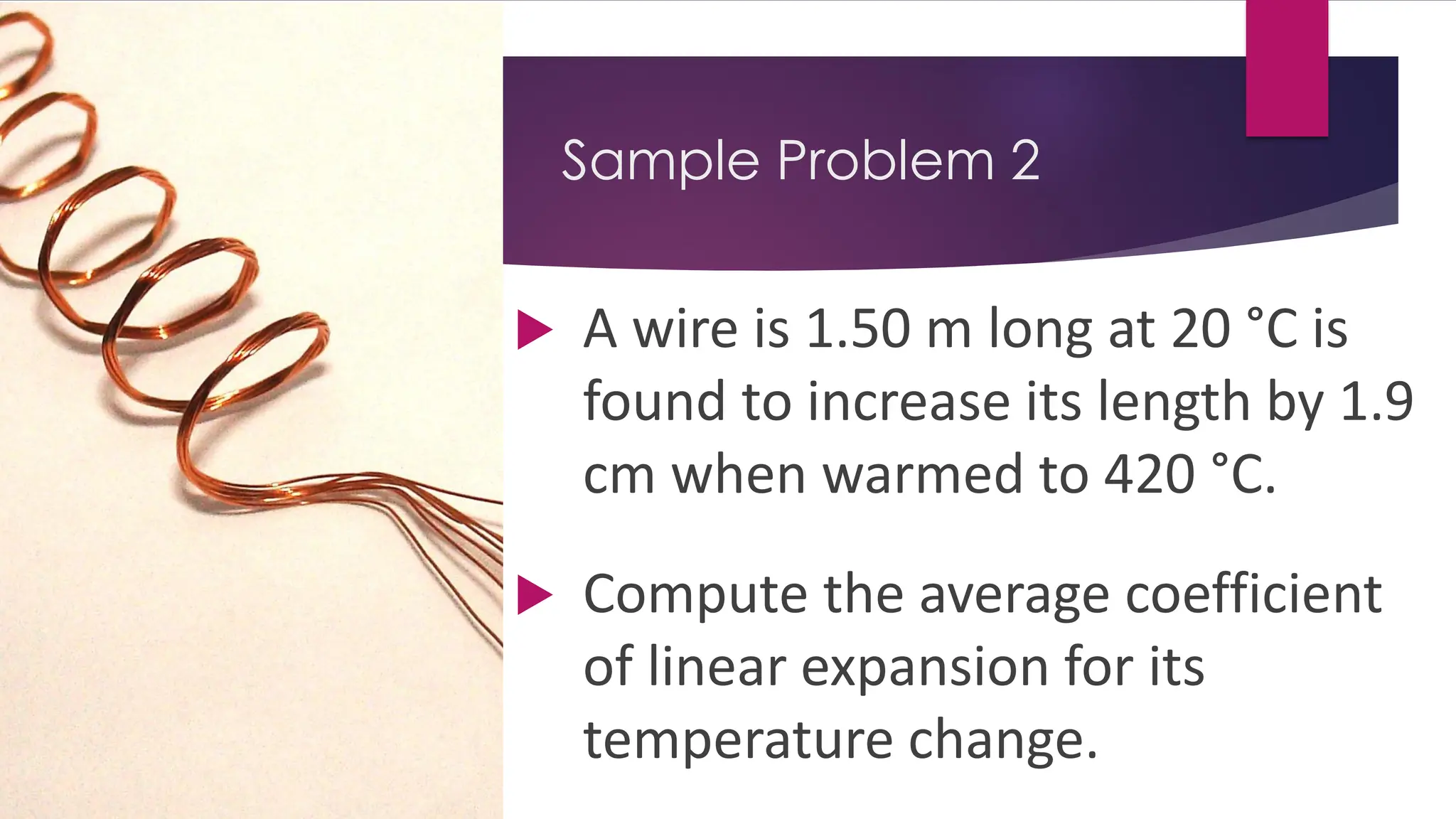
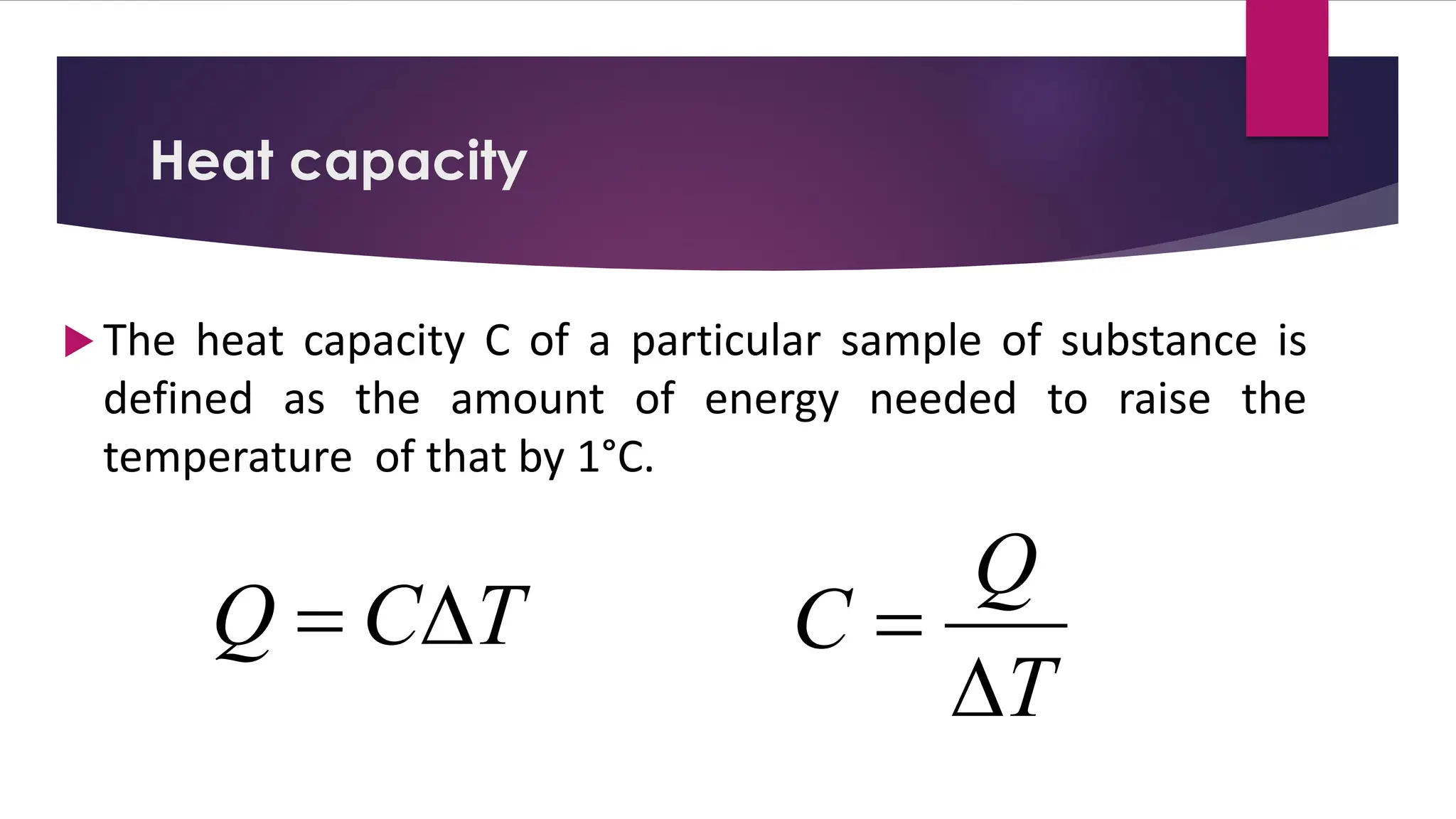
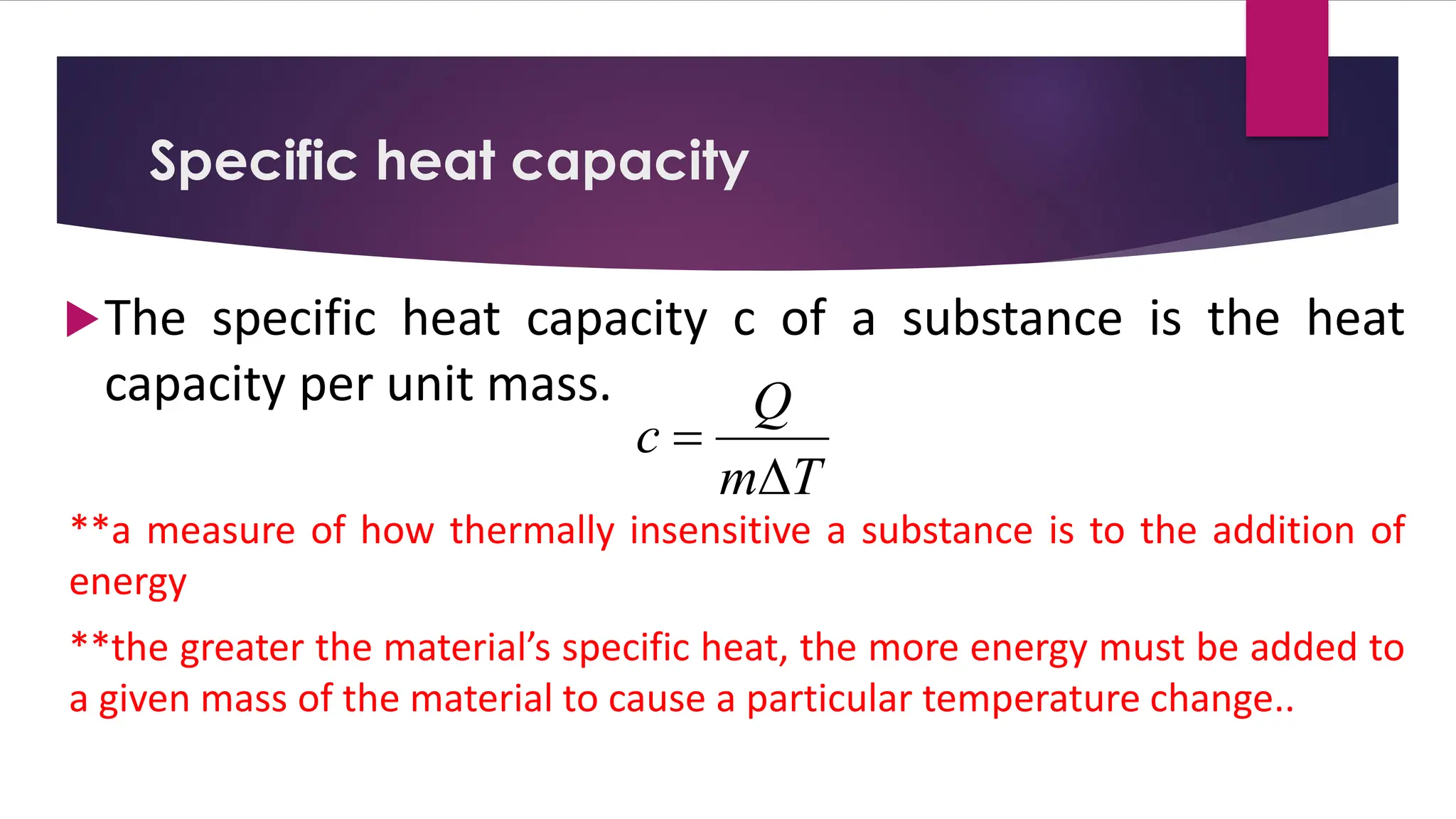
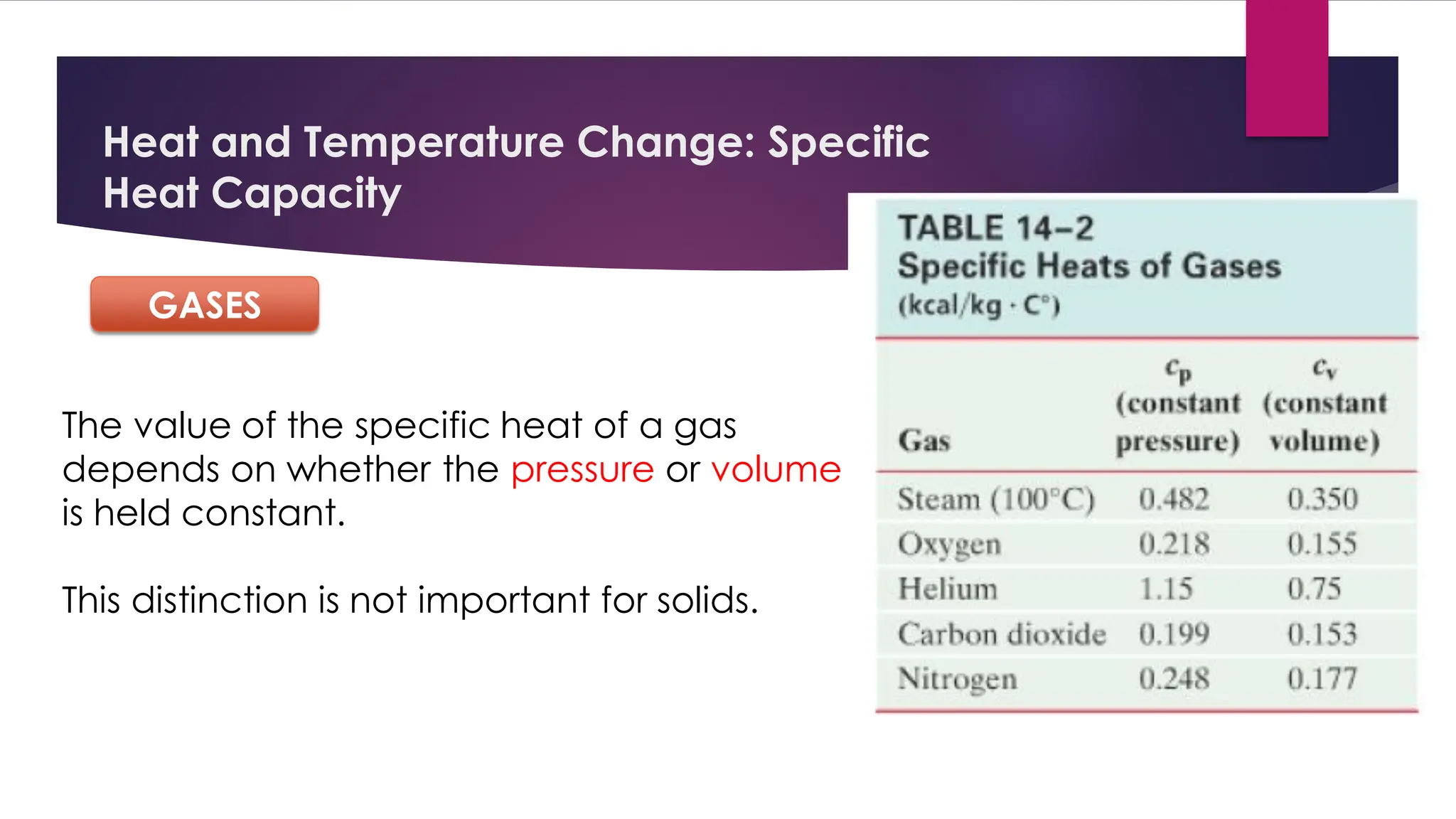
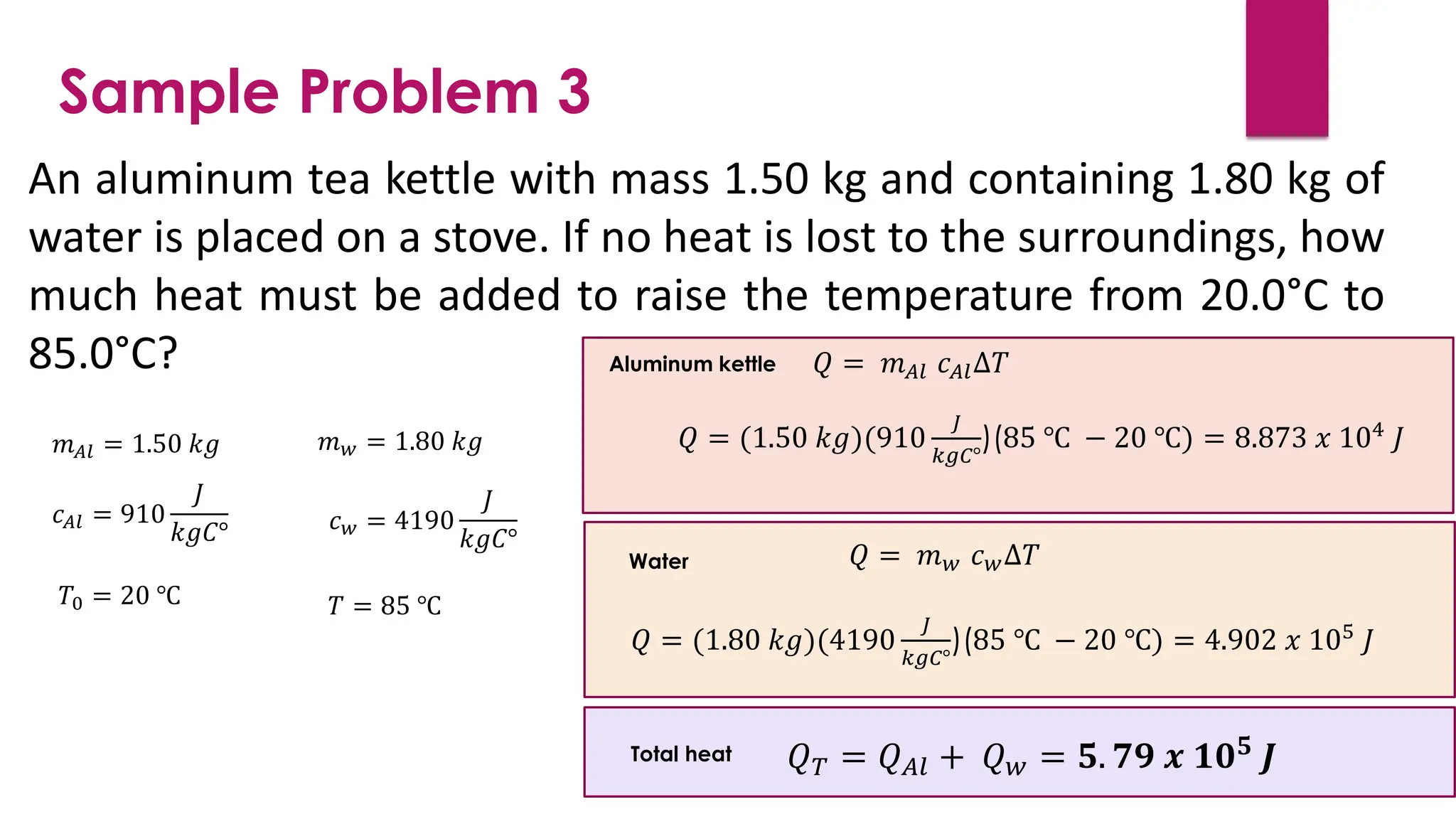
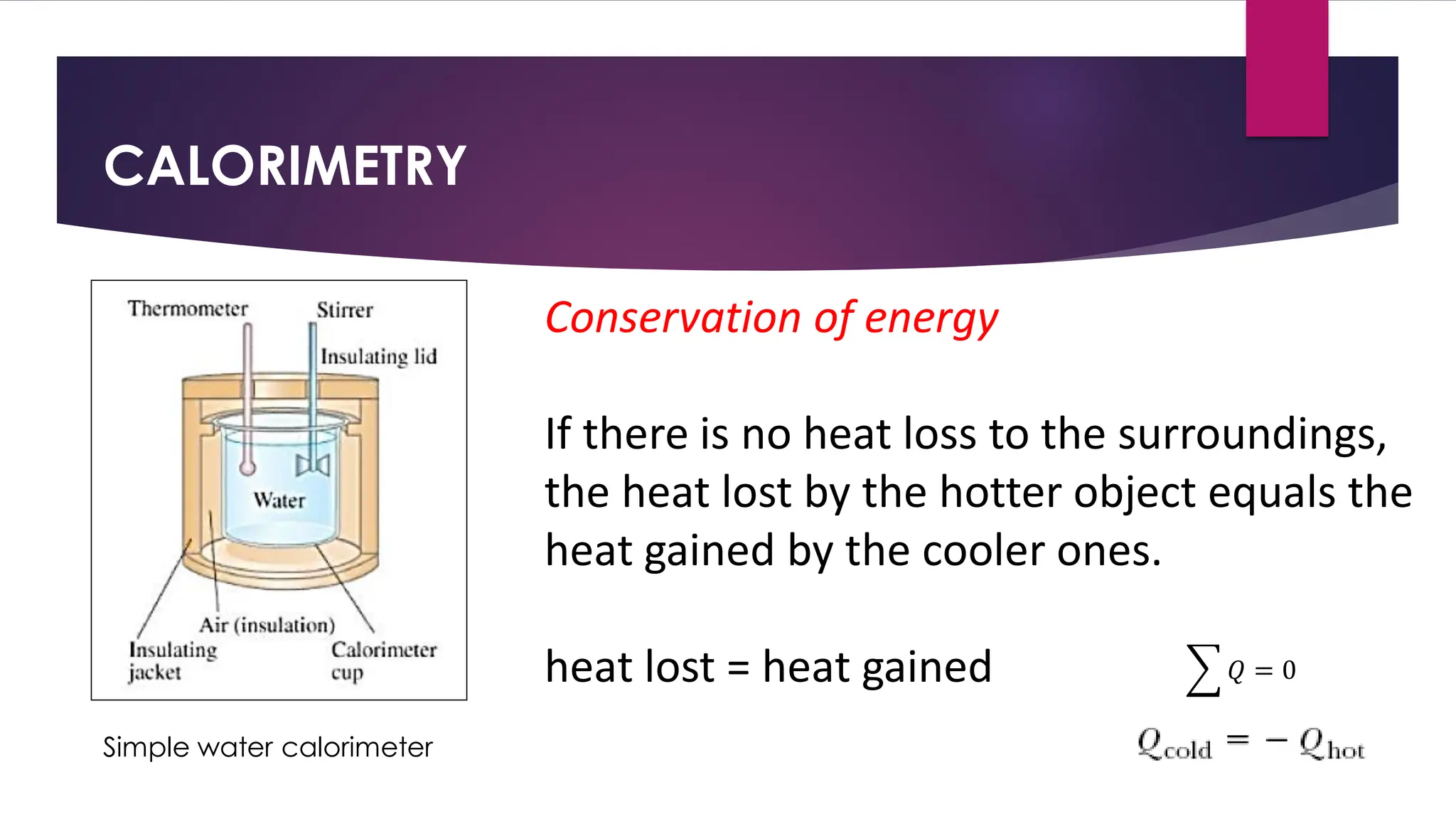
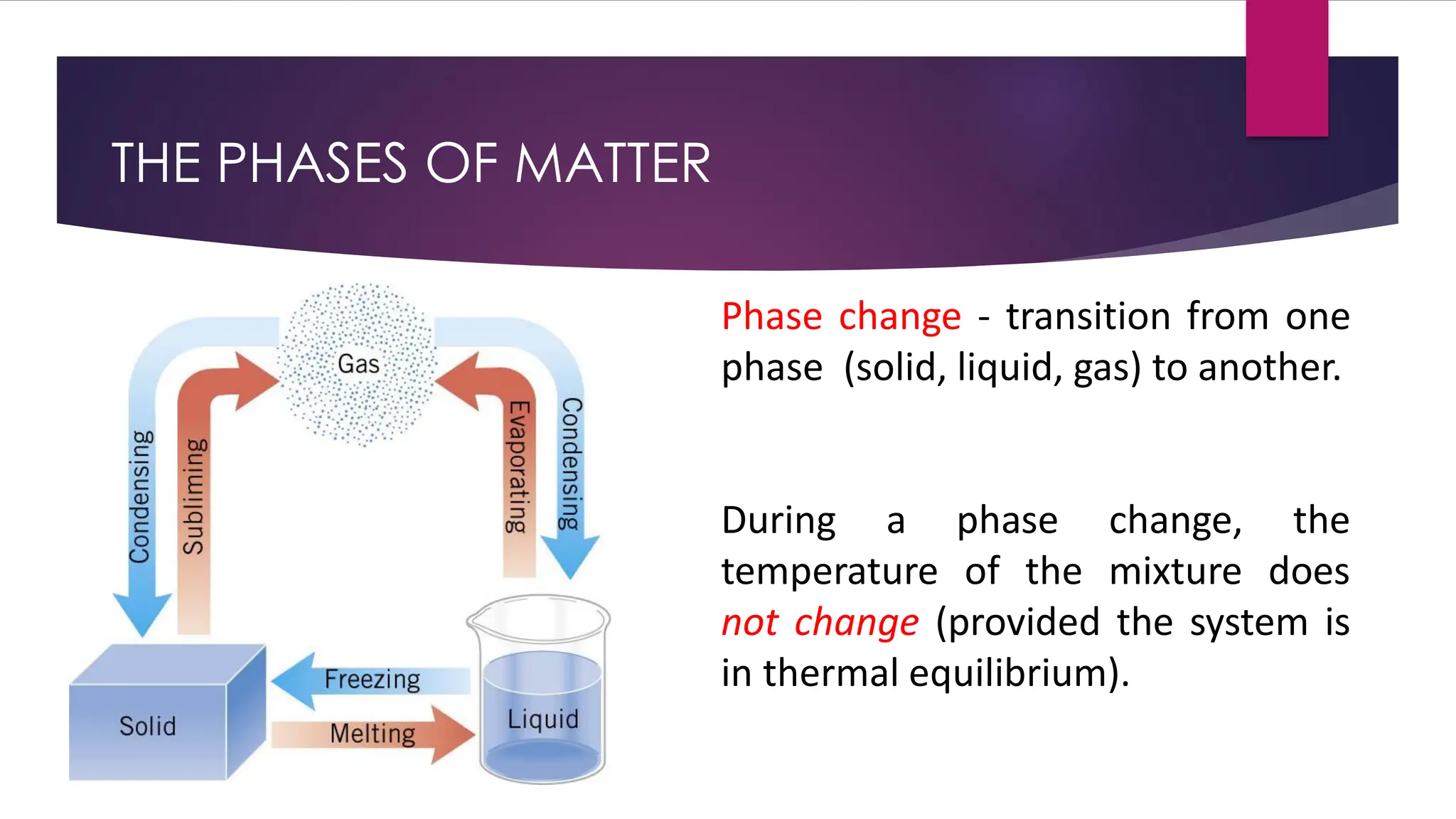
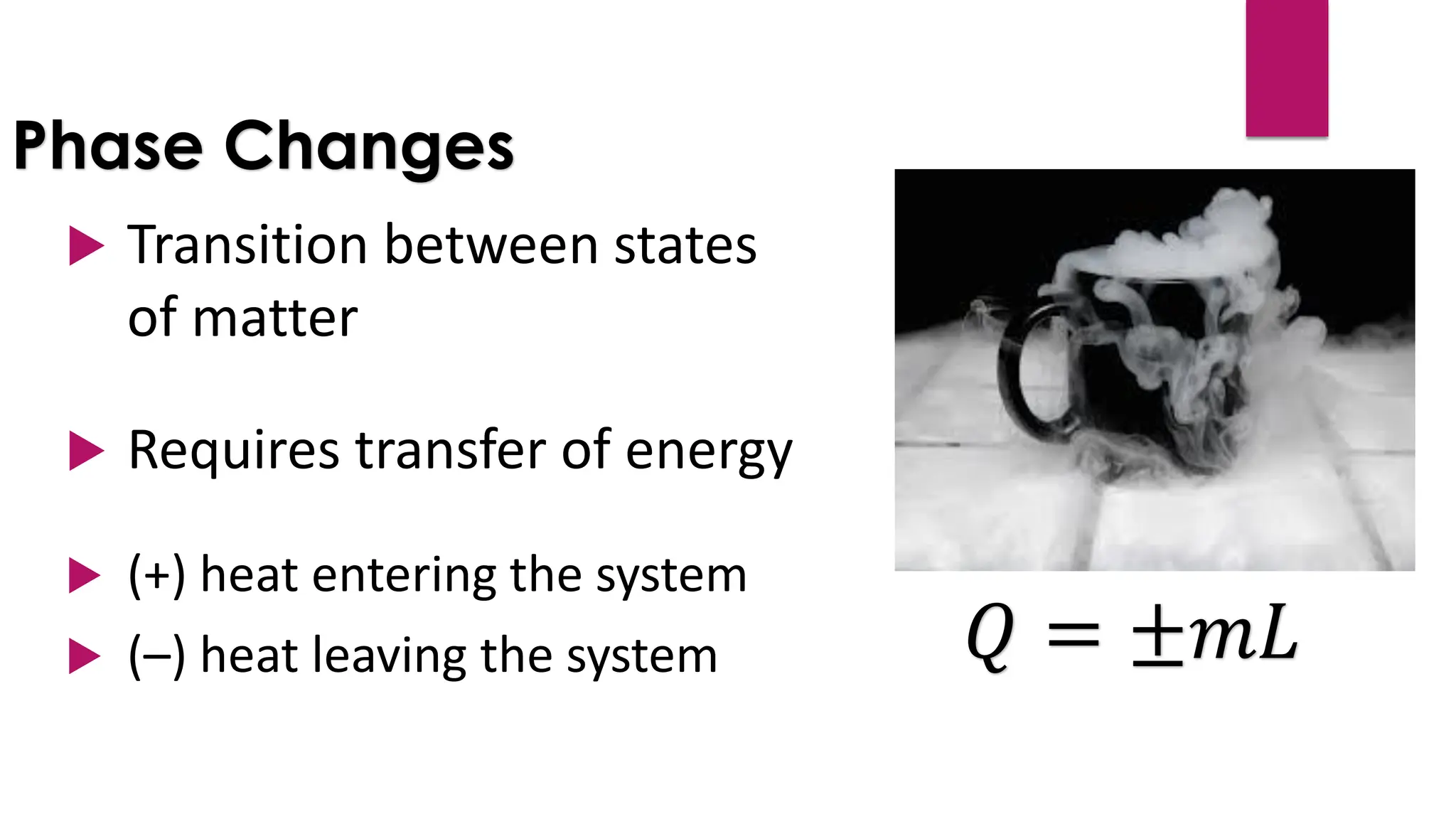
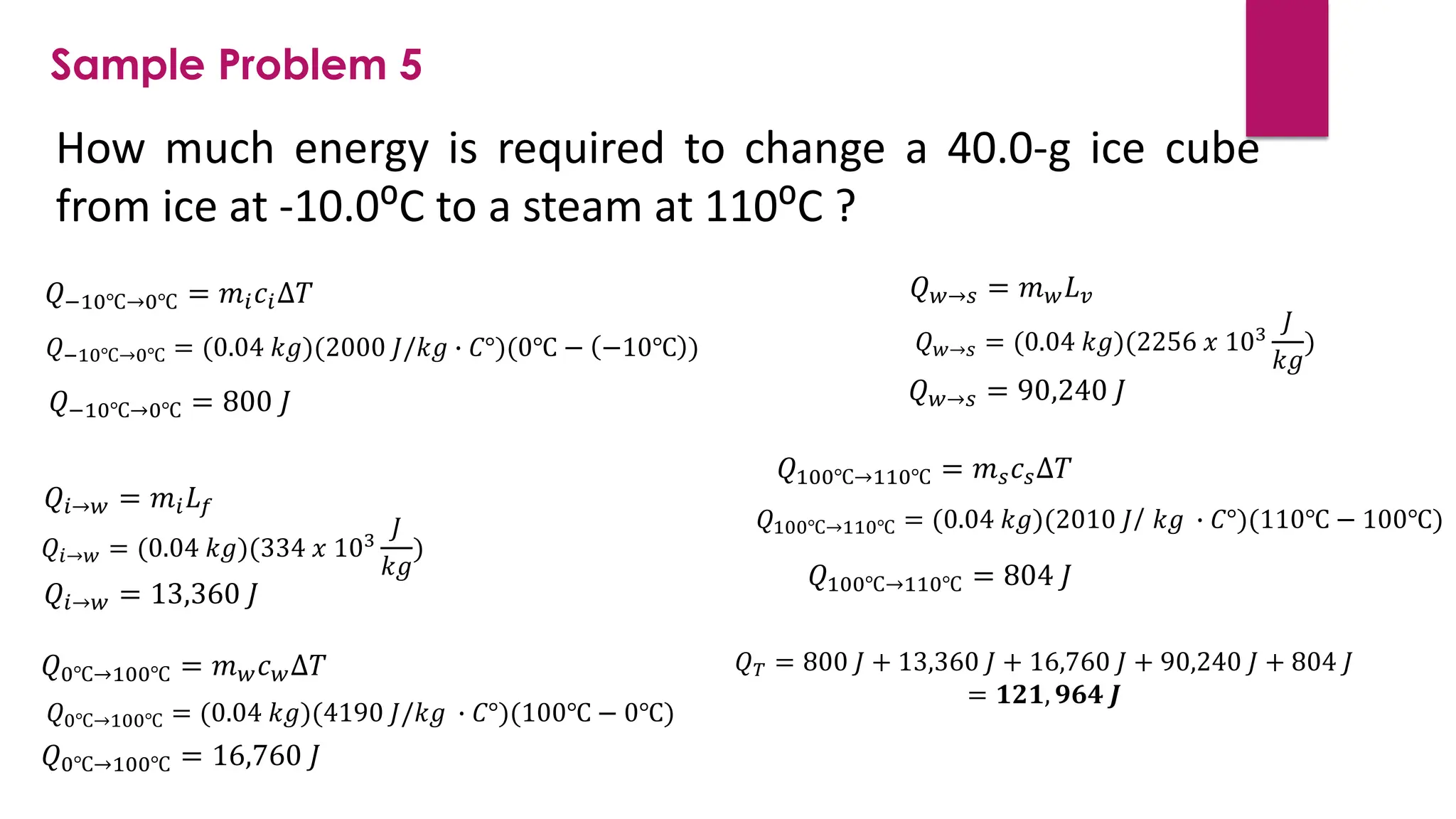
The document provides an overview of temperature and heat, including definitions, measurement methods, and the behavior of matter in different states related to thermal dynamics. It explains the zeroth law of thermodynamics, temperature scales, thermal expansion, heat transfer, and calorimetry. There are also sample problems to illustrate the calculations involved in determining specific heat and thermal energy changes during phase transitions.