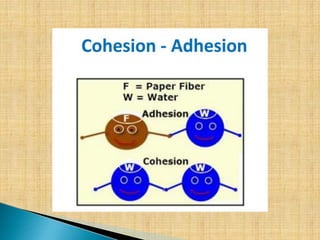
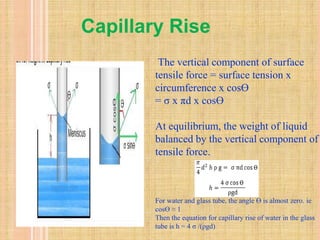
Capillarity refers to the ability of a liquid to flow in narrow spaces due to intermolecular forces between the liquid and surrounding solid surface. Capillarity occurs due to the balance between cohesive forces within the liquid and adhesive forces between the liquid and surrounding surface. There are two types of capillarity: capillary rise, where adhesion is greater than cohesion causing liquid to flow upward; and capillary fall, where adhesion is less than cohesion causing liquid to flow downward. The height of capillary rise or fall can be calculated based on properties of the liquid and surrounding material. Common examples of capillarity include the wicking of oil up a lamp wick and the absorption of ink into blotting paper.