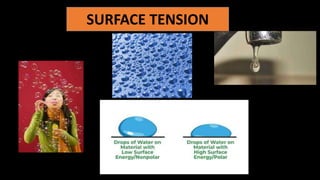
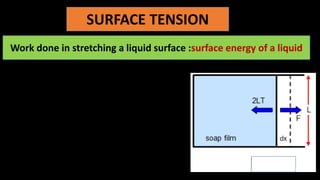
The document discusses surface tension, highlighting factors that affect it such as temperature, impurities, and the medium in contact. It explains the concept of capillarity and the behavior of liquids like water and mercury in relation to solid surfaces, specifically detailing the angle of contact. Additionally, it contrasts cohesive and adhesive forces, emphasizing their relevance to the phenomenon of surface tension.