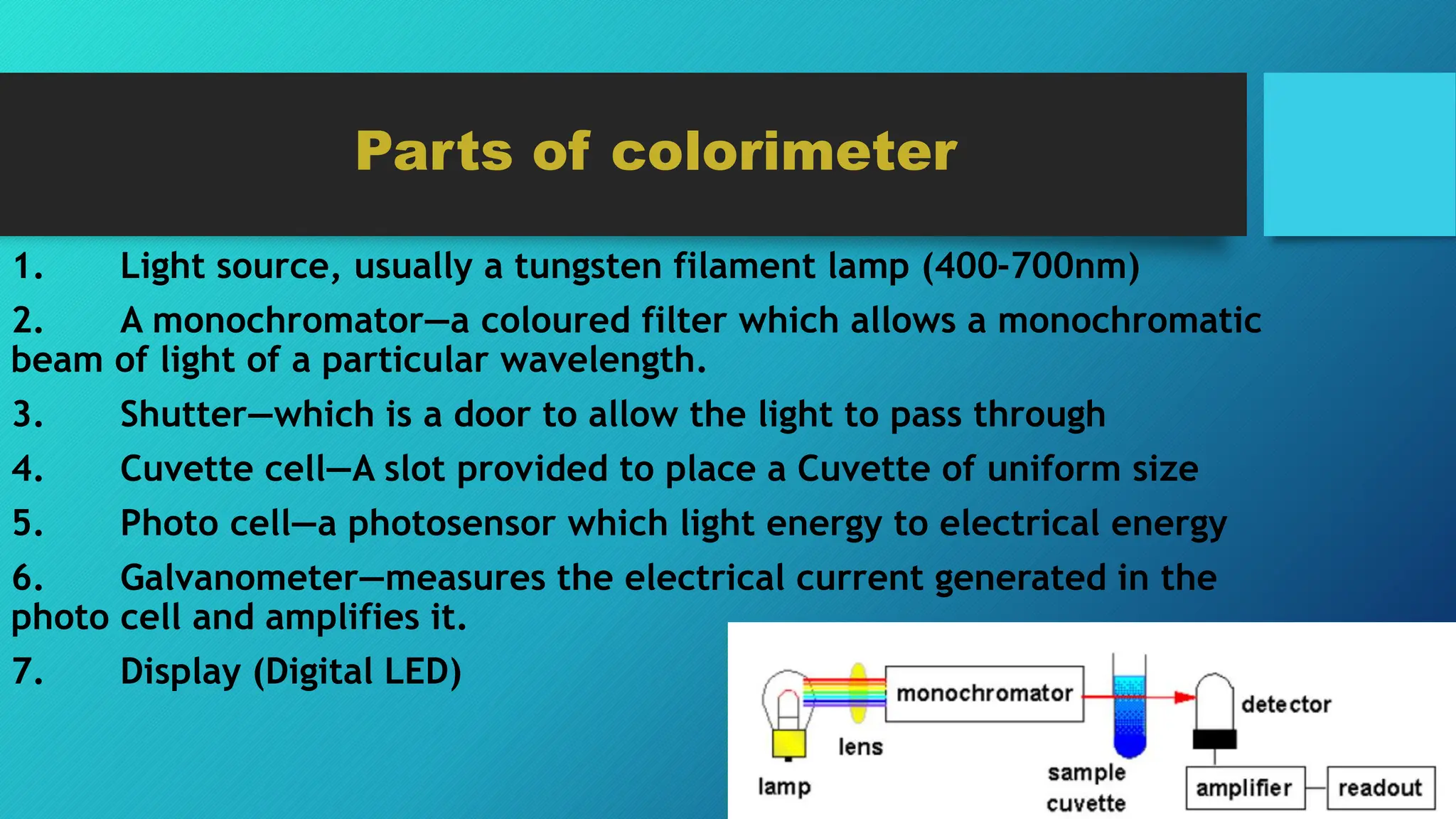
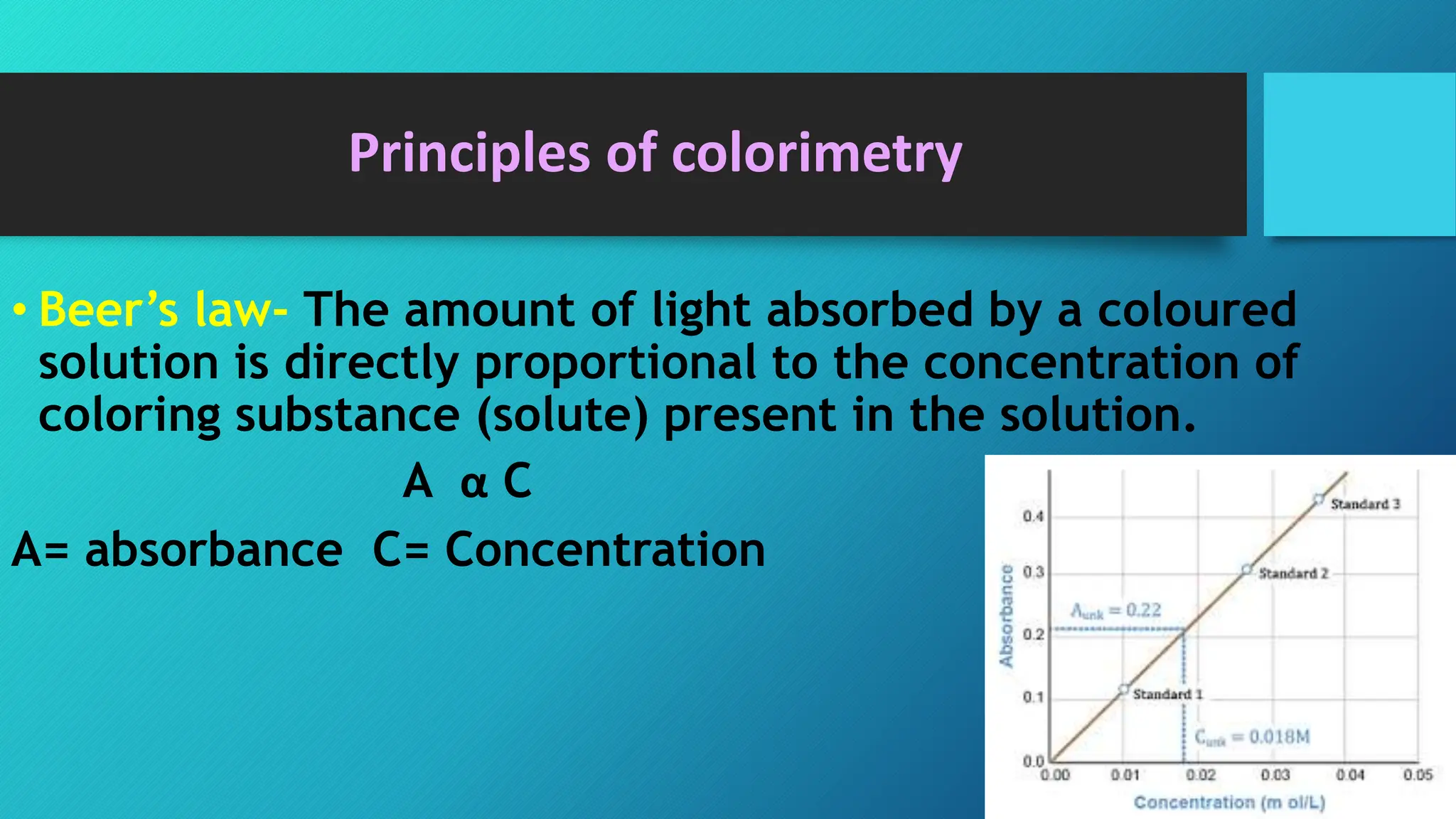
The document discusses colorimetry, including the principles of how colors are perceived and the function of a colorimeter, which measures the intensity of color based on light absorption and transmittance. It explains key concepts such as Beer-Lambert Law and outlines the steps for using a colorimeter, preparing standard curves, and conducting quantitative experiments. Additionally, it compares colorimeters to spectrophotometers, highlighting differences in sensitivity and measurement range.