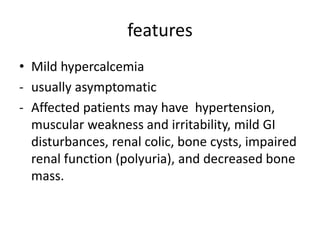
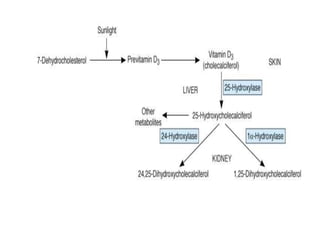
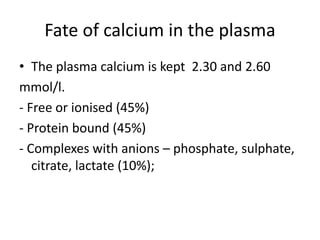
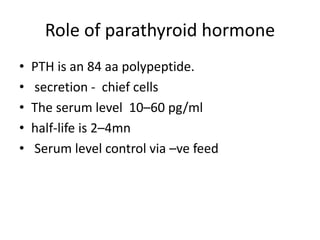
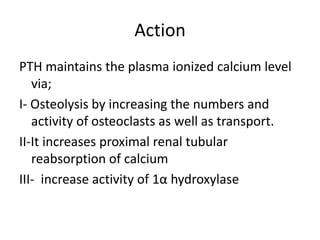
Calcium metabolism involves three tissues (bone, intestine, kidney), three hormones (PTH, calcitonin, vitamin D), and three cell types that maintain normal calcium levels. Calcium is important for bone health, muscle function, and other processes. The daily requirement is 400-500mg for adults. PTH and calcitonin work to maintain calcium within normal ranges in plasma. Hypocalcemia can cause tetany and hypercalcemia can damage organs if severe. Tests are used to diagnose and treat imbalances.







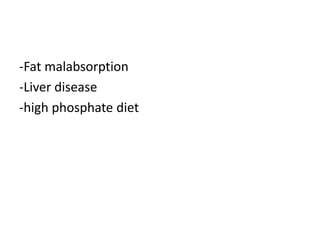


![Calcium correction
• Corrected ca2+ (mmol/l)= (Ca) + 0.02 (40 -
albumin)
• Corrected calcium (mg/dL) = Ca (mg/dL) + 0.8
(4.0 - serum albumin [g/dL]),](https://image.slidesharecdn.com/calciummetabolism-170322063739/85/Calcium-metabolism-18-320.jpg)