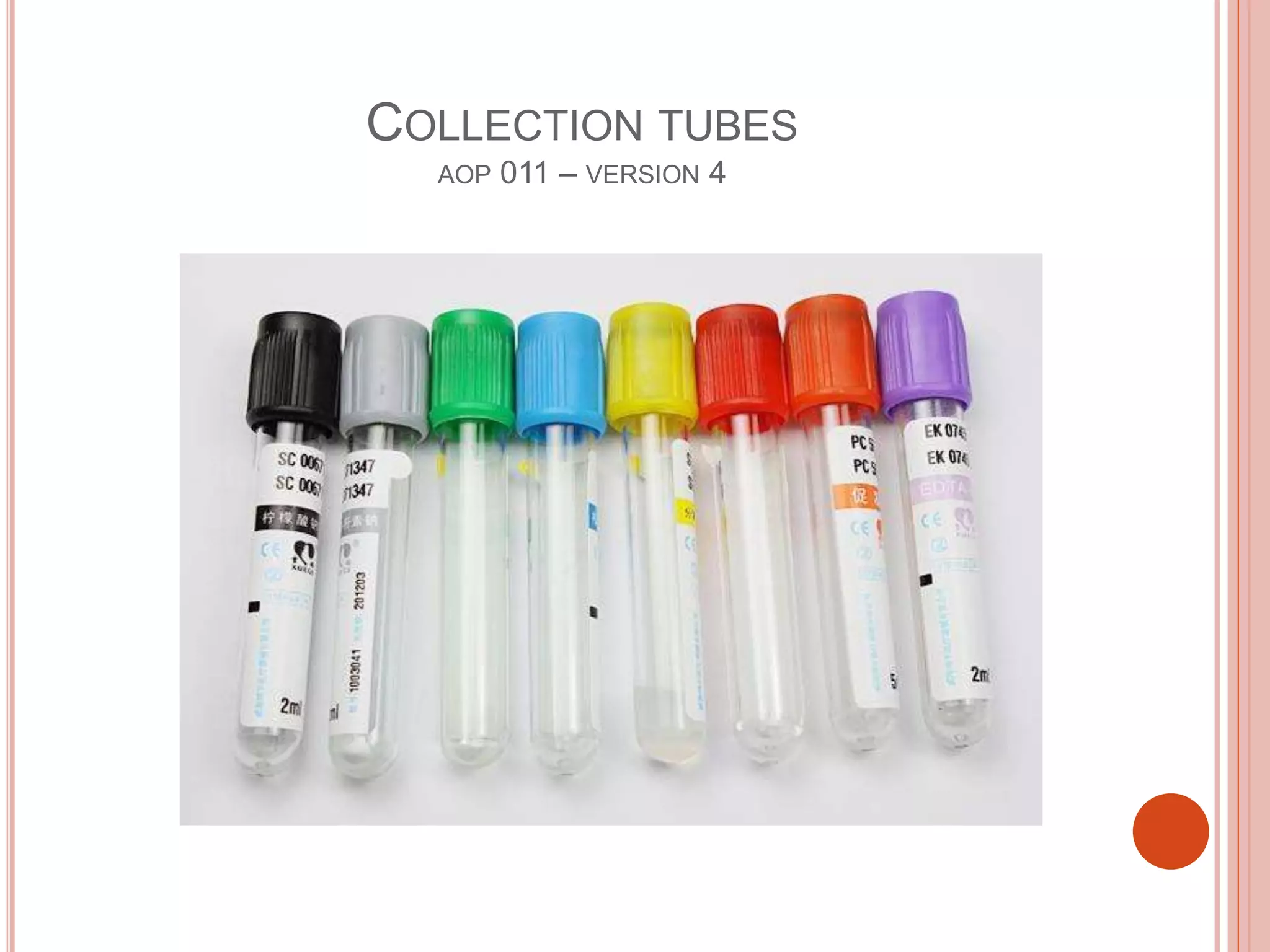
This document outlines policies and procedures for blood specimen collection and disposal of sharp objects at KFCH. It defines venipuncture and different types of collection tubes used, as well as nursing responsibilities for blood collection and labeling of samples. The standard operating procedure for disposal of sharp objects specifies using puncture-proof containers labeled as biohazardous waste and proper handling, transport, and disposal of filled sharp containers. Recommendations include strictly following policies to ensure staff and patient safety during phlebotomy and waste disposal.