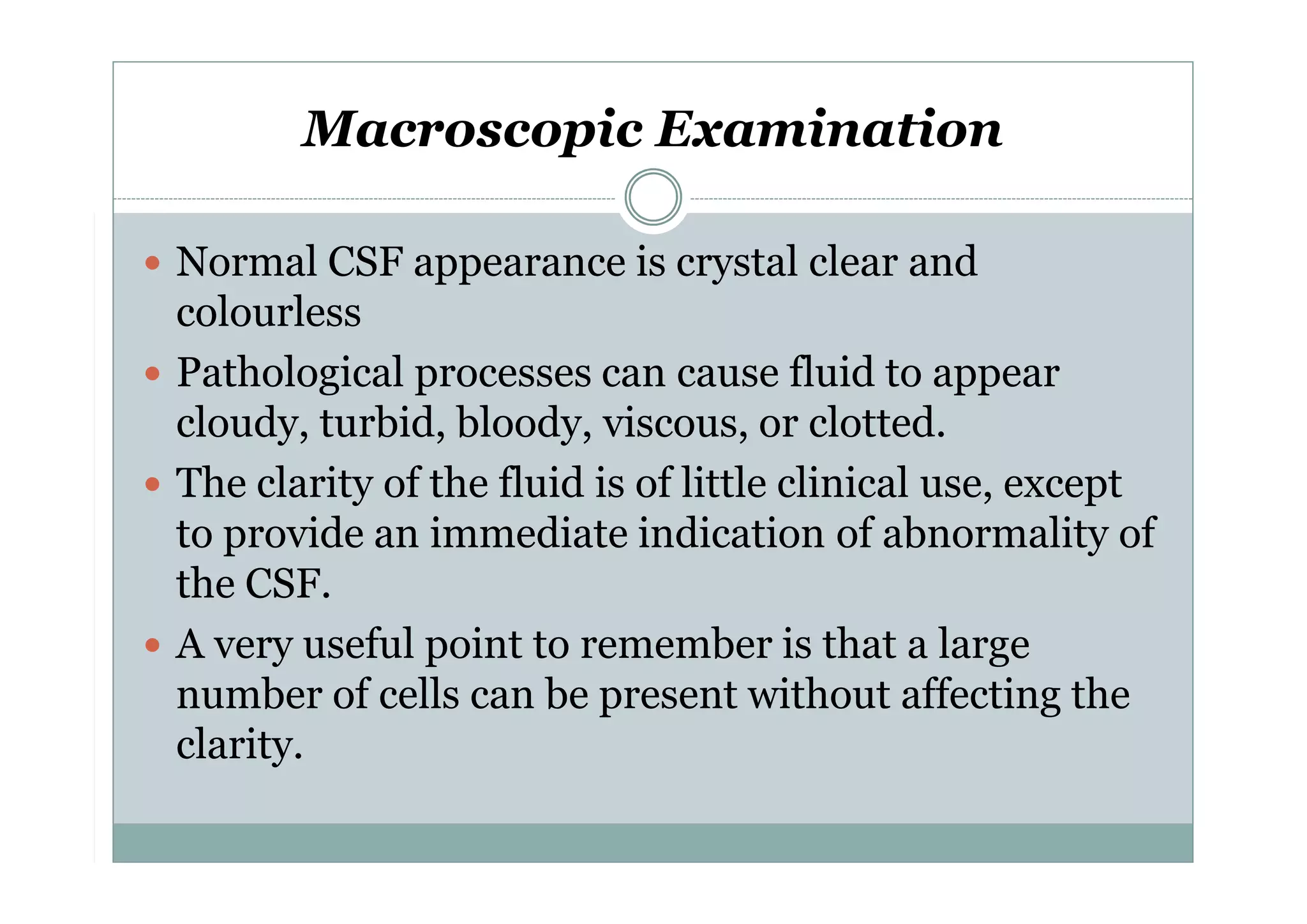
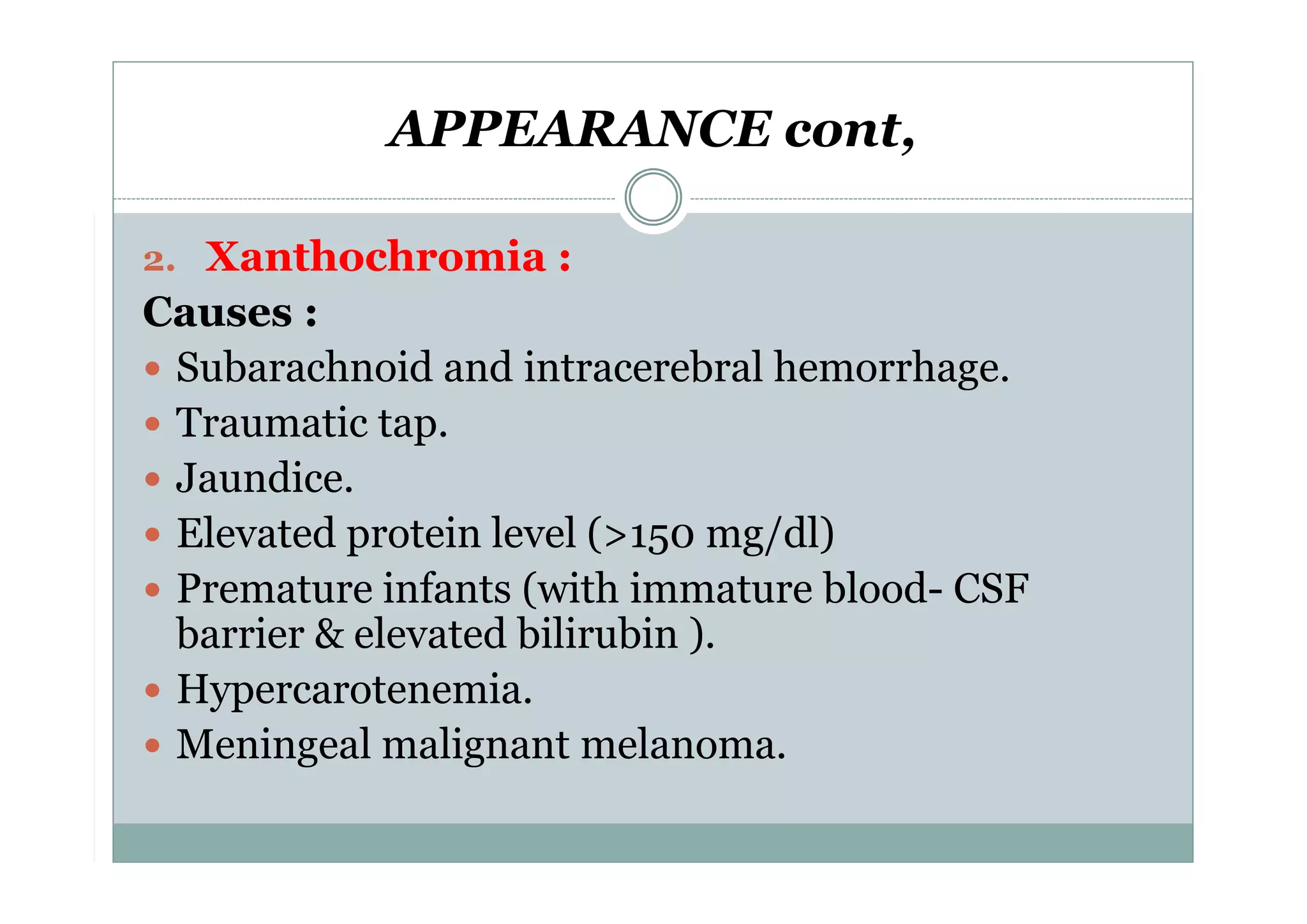
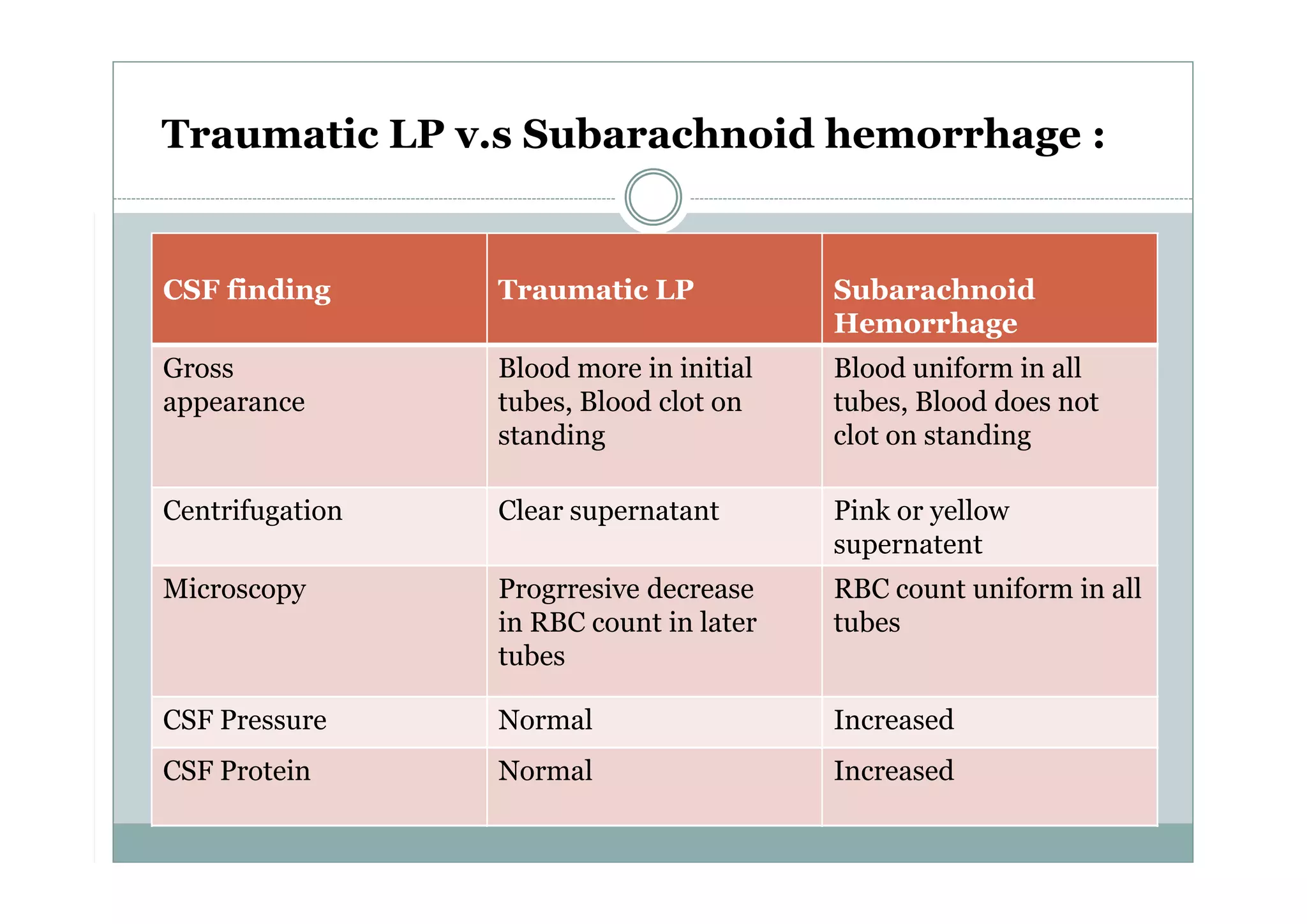
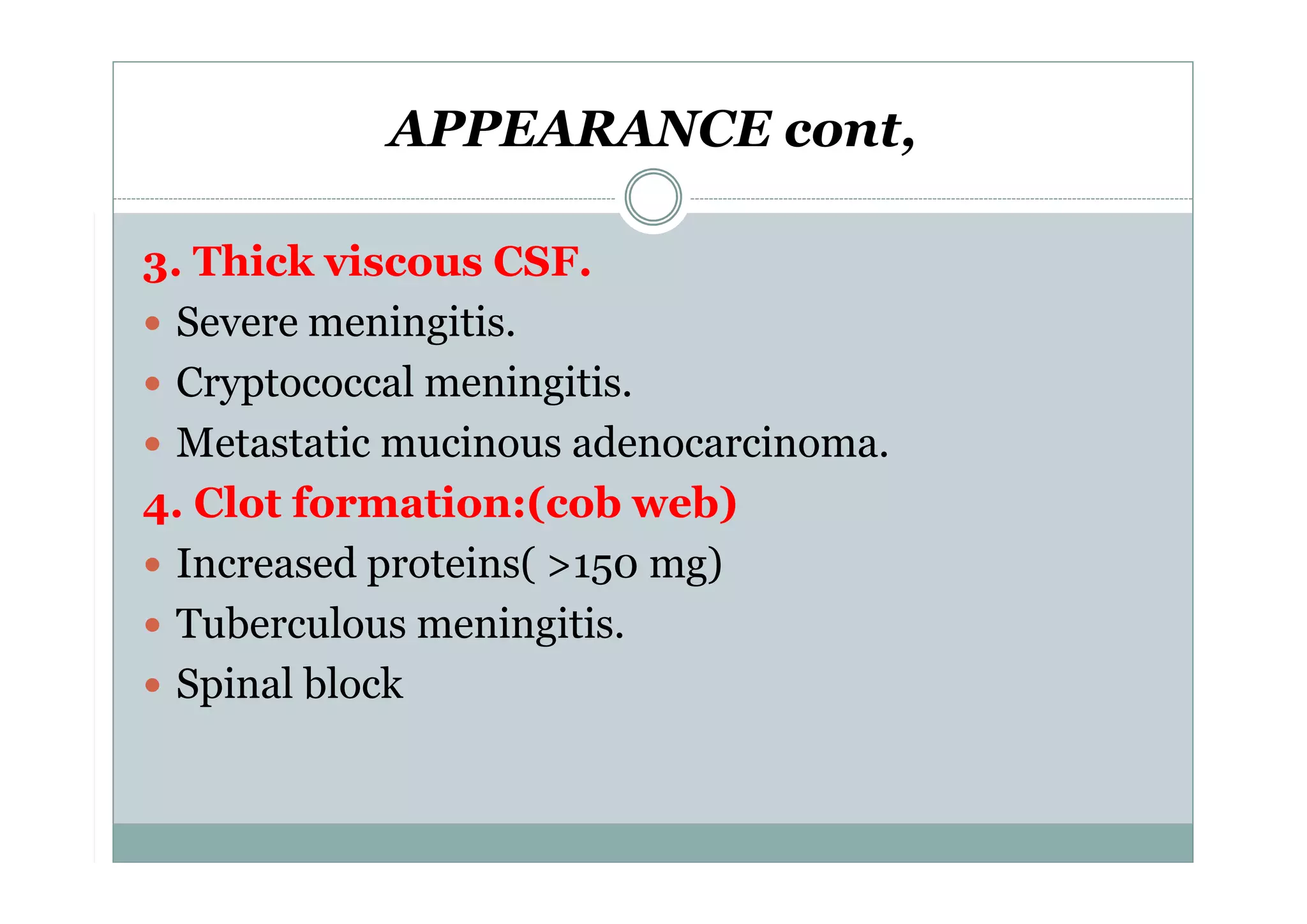
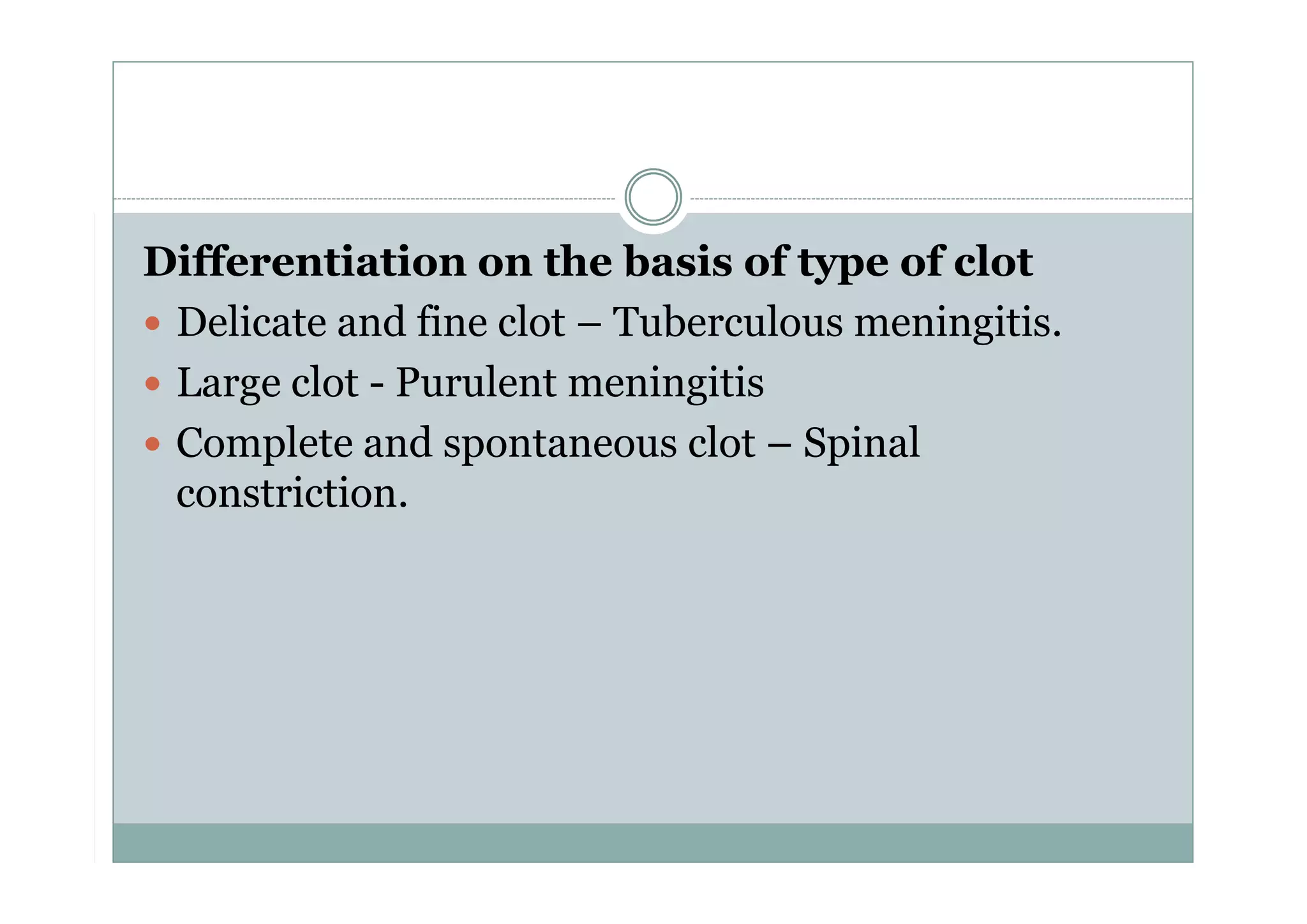
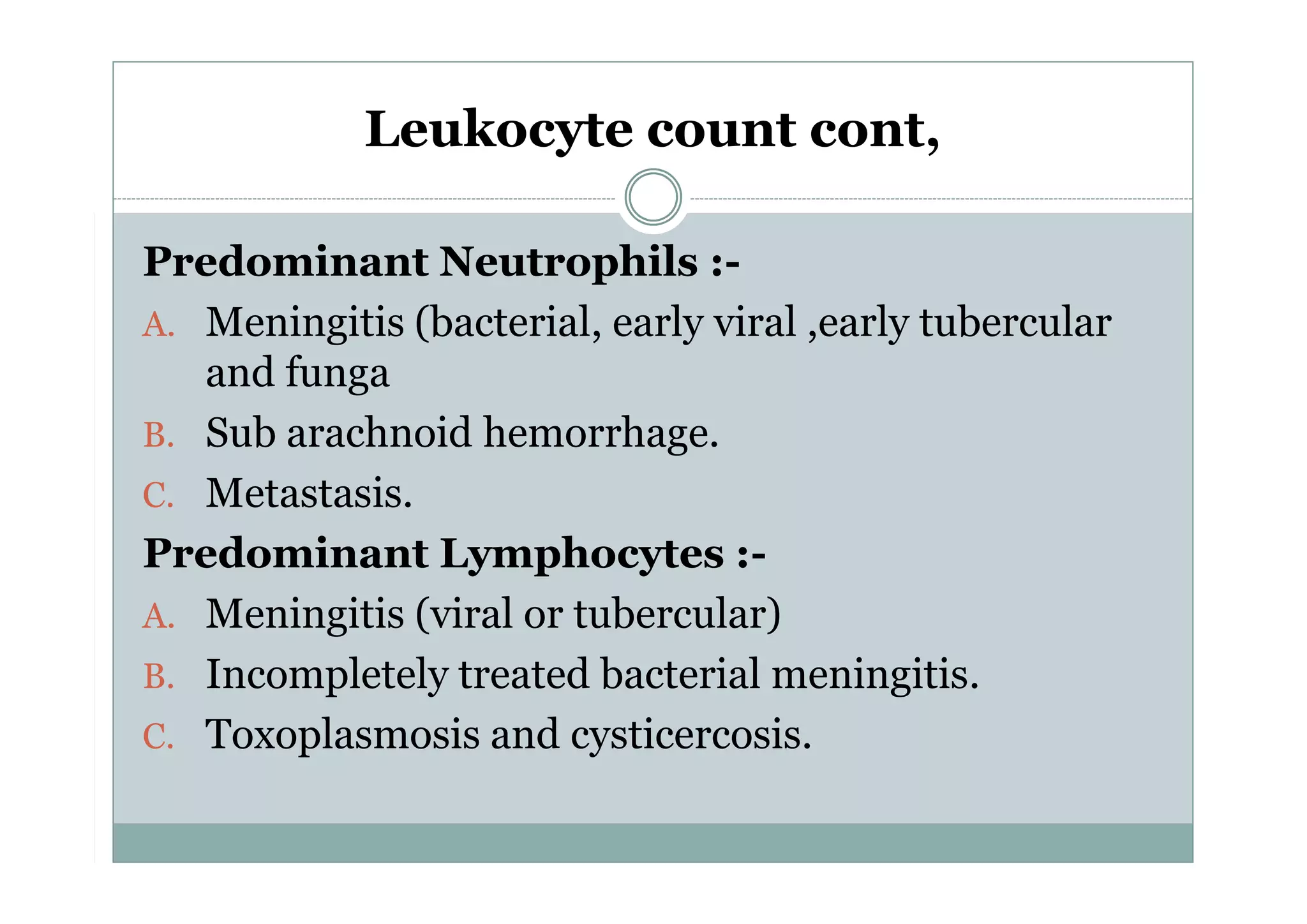
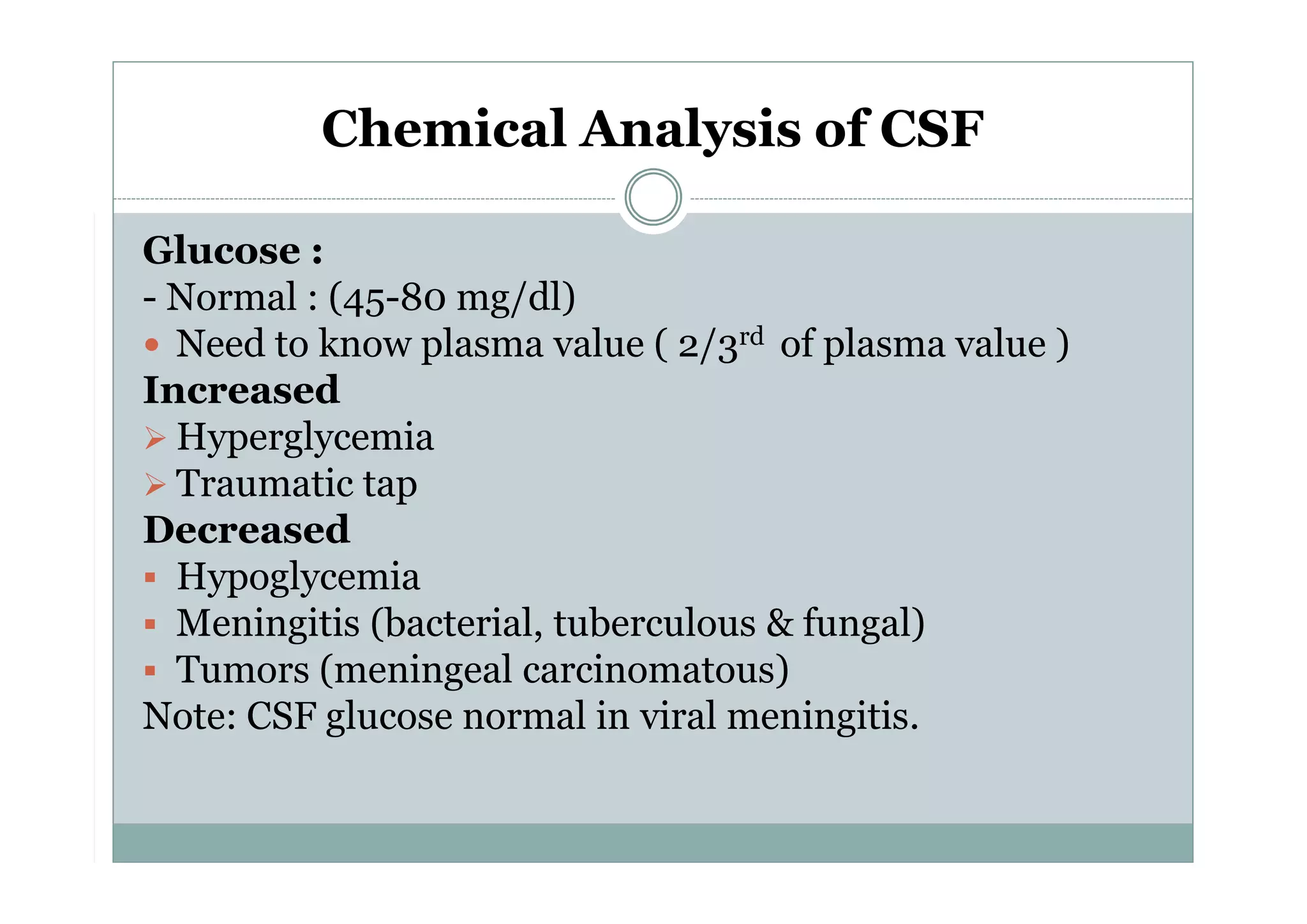
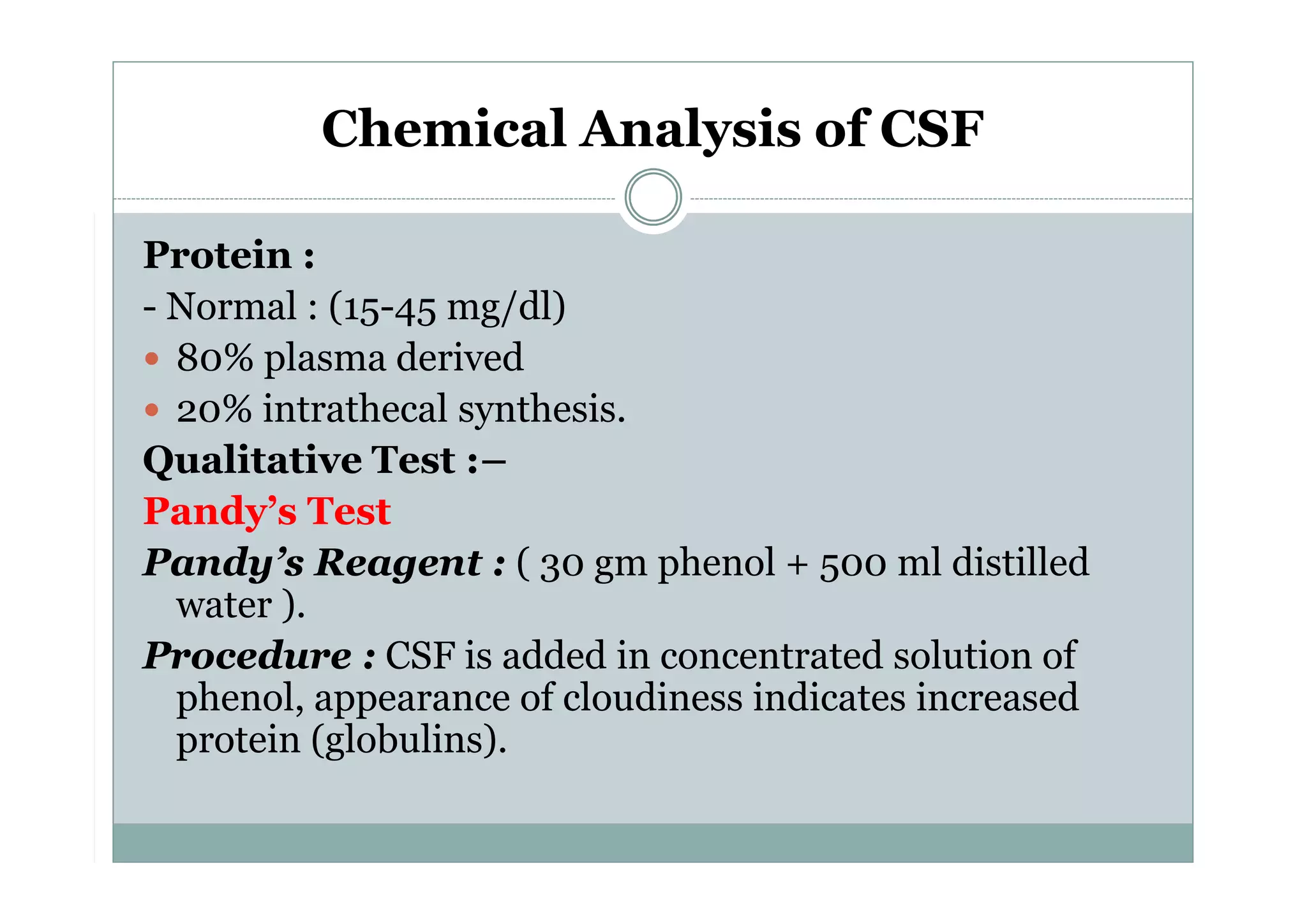
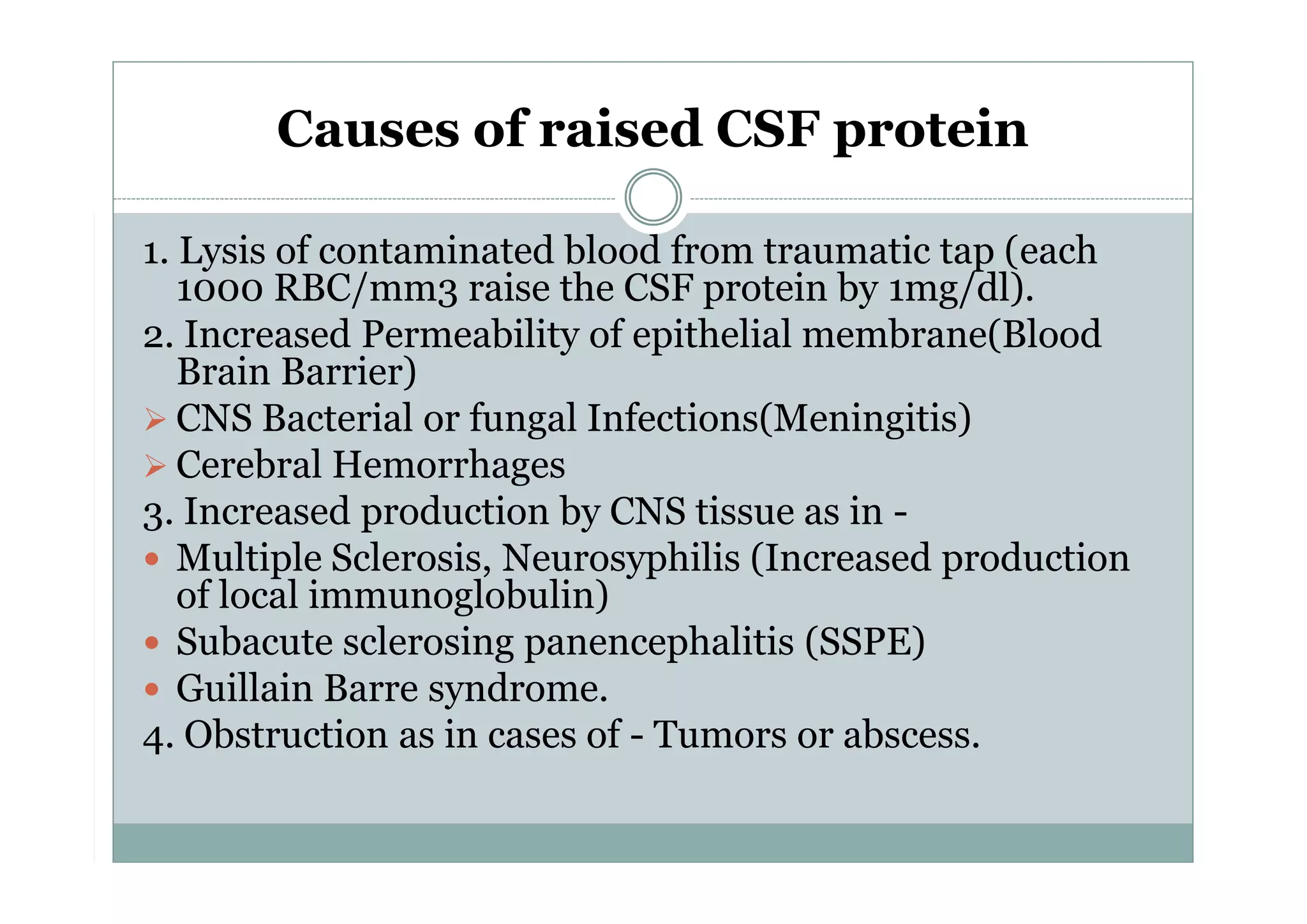
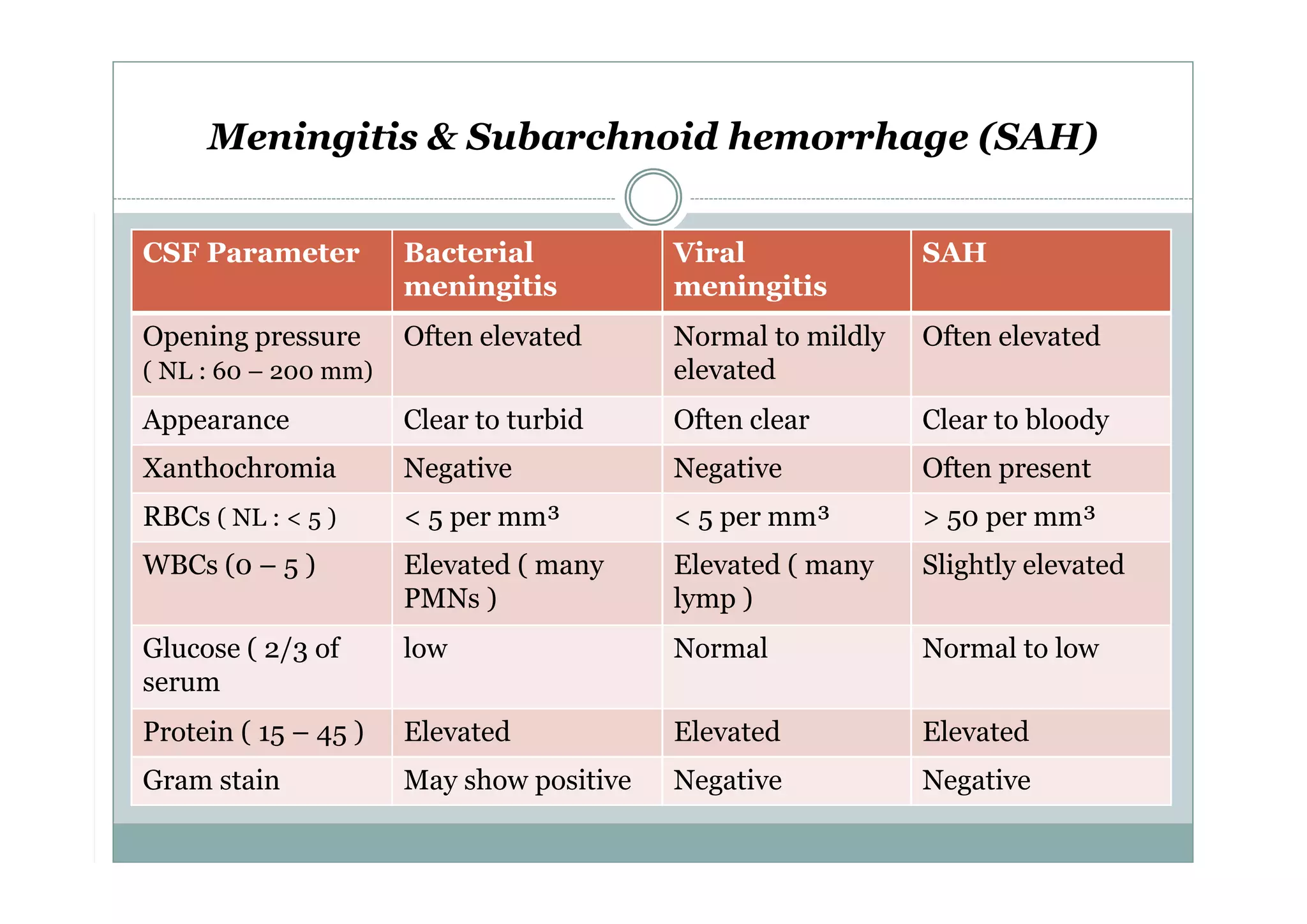
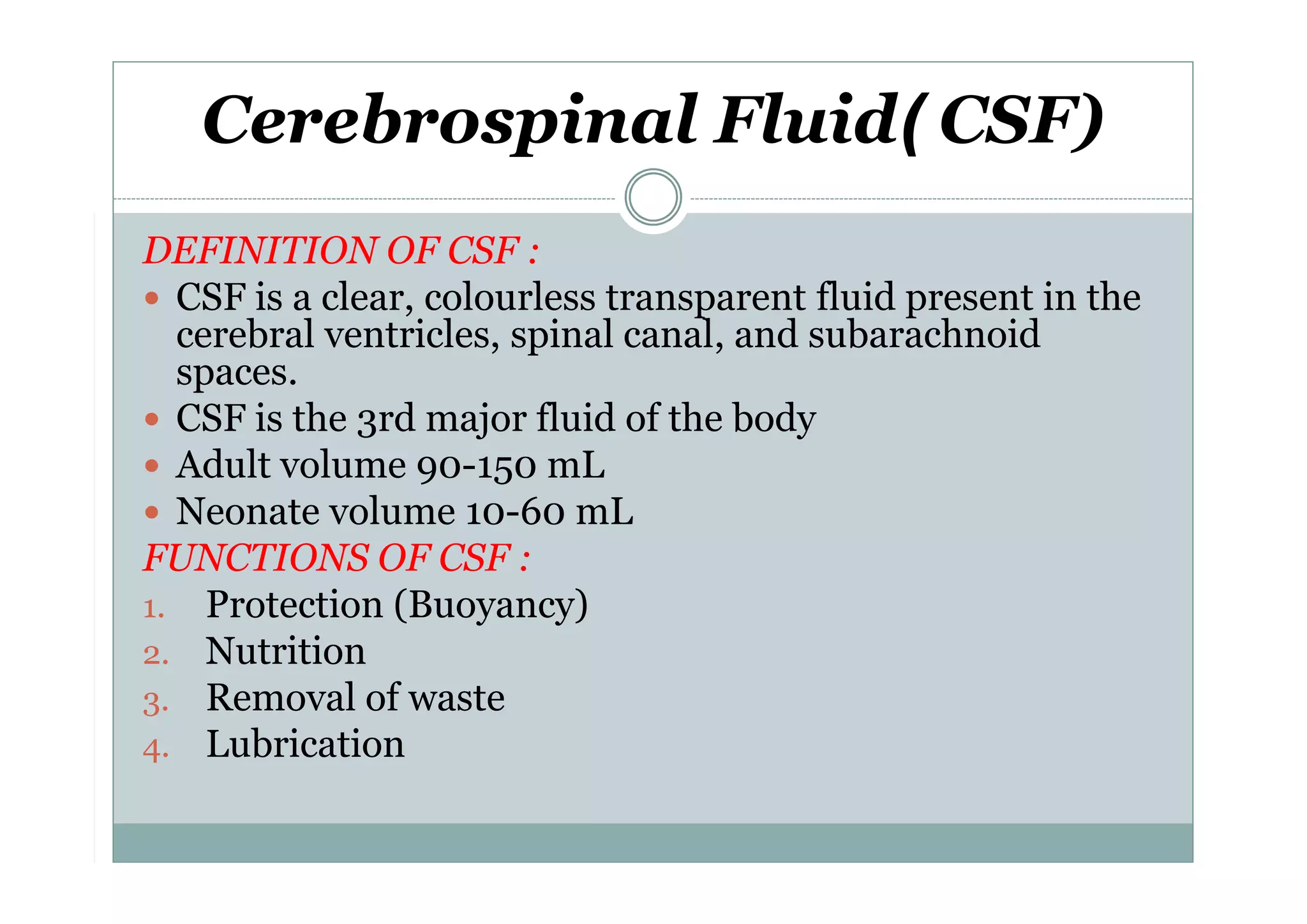
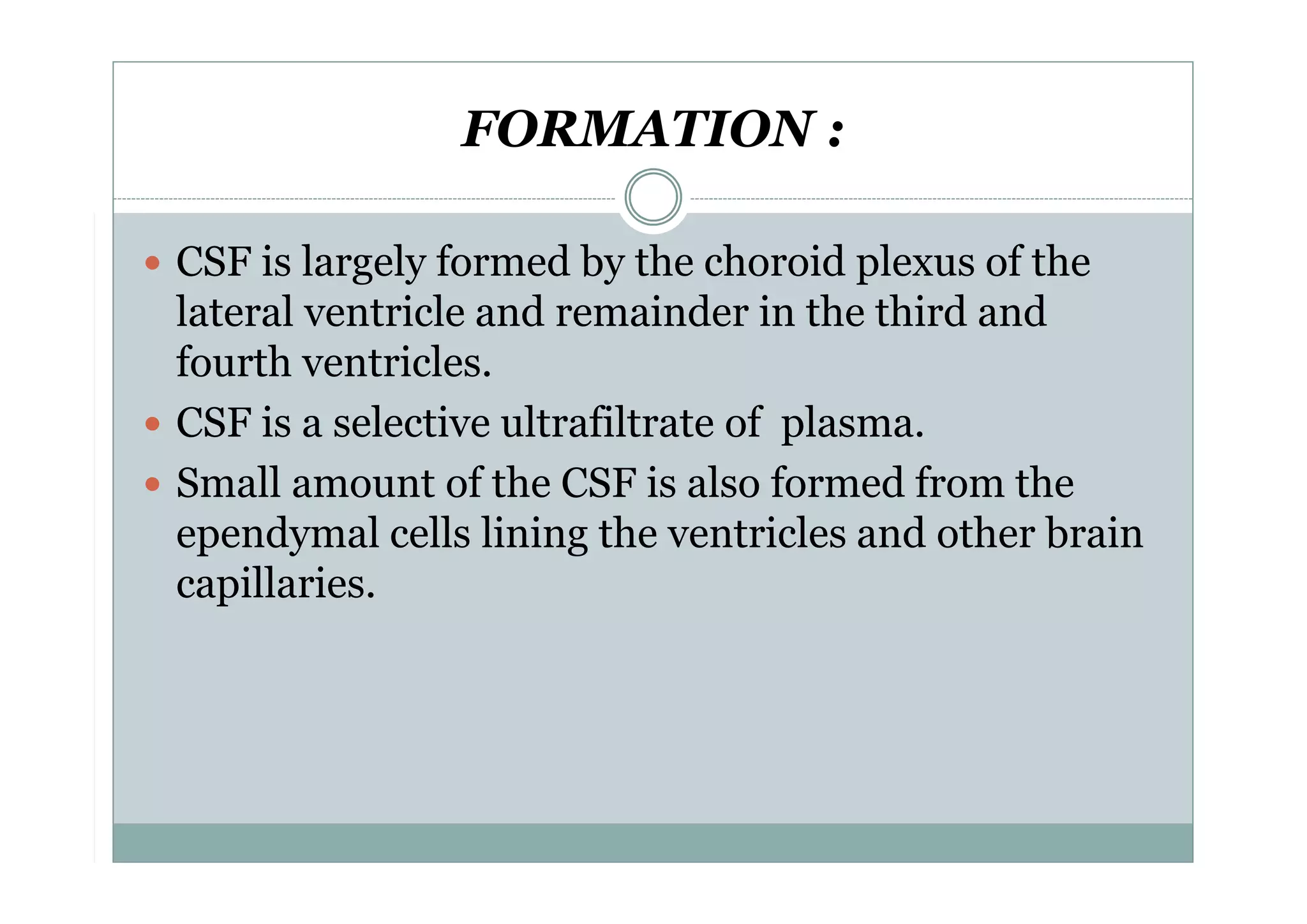
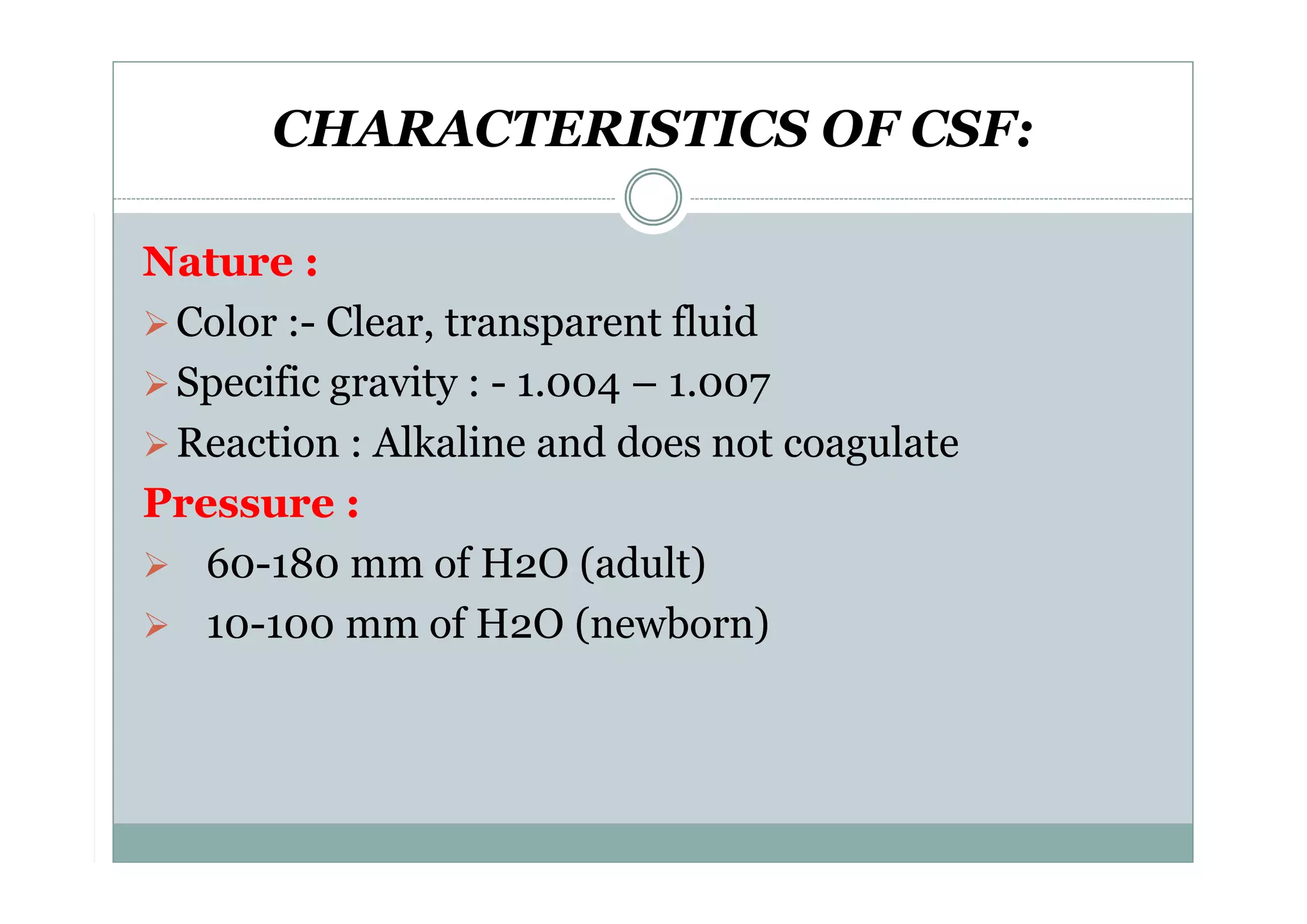
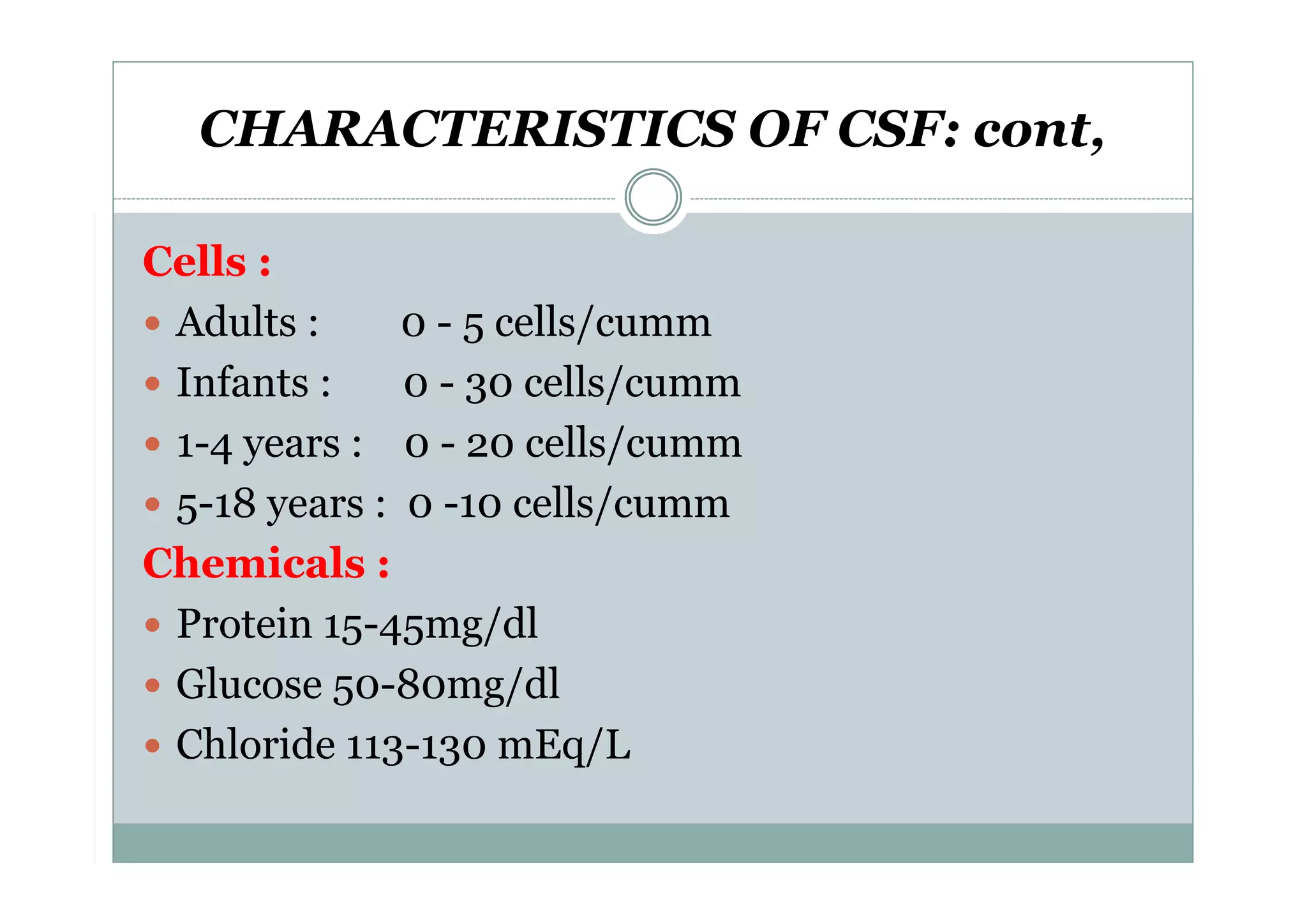
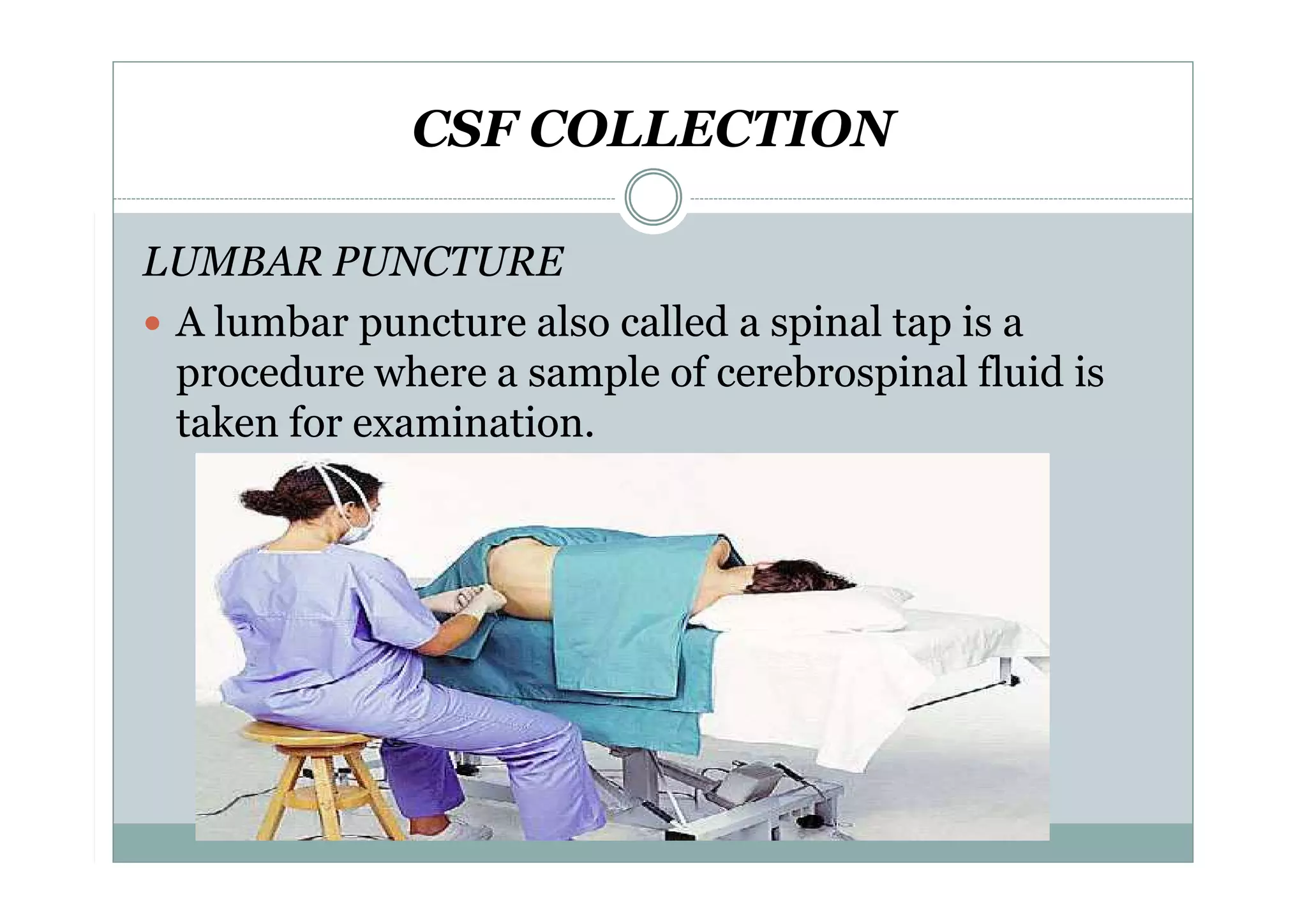
CSF is a clear fluid found in the brain and spinal cord that serves several functions, including protection, nutrition, and waste removal. It is produced by the choroid plexus at a rate of 20 mL/hour in adults. Normal CSF is clear and colorless with a protein level of 15-45 mg/dL and glucose level of 50-80 mg/dL. Examination of CSF includes analysis of appearance, cell count, chemistry, and cytology to diagnose conditions like meningitis or hemorrhage. Increased cells typically indicate infection or inflammation while decreased glucose suggests bacterial or fungal meningitis.



![ROUTINE & OPTIONAL CSF TESTS
Routine :
Gross examination
Cell Counts + Differential
Glucose [60-70% plasma]
Protein [15 – 40 mg/dL]
When Indicated :
Cultures
Stains [Gram, Acid Fast]
Cytology
Electrophoresis
VDRL
Fungal and bacterial antigen
D –Dimers
PCR
Routine :
Gross examination
Cell Counts + Differential
Glucose [60-70% plasma]
Protein [15 – 40 mg/dL]
When Indicated :
Cultures
Stains [Gram, Acid Fast]
Cytology
Electrophoresis
VDRL
Fungal and bacterial antigen
D –Dimers
PCR](https://image.slidesharecdn.com/cerebrospinalfluid1-200621093043/75/Cerebrospinal-fluid-sample-collection-9-2048.jpg)