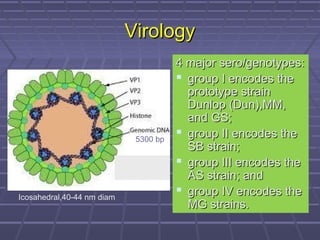
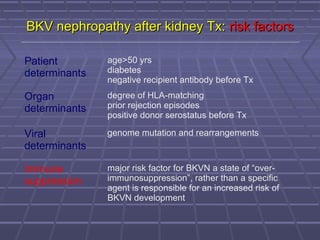
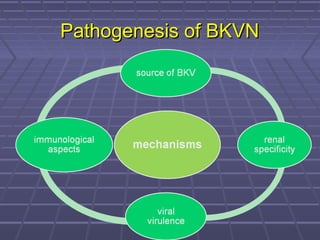
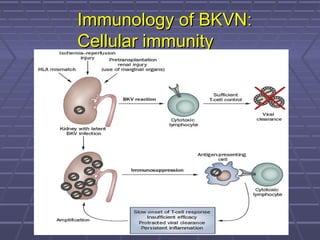
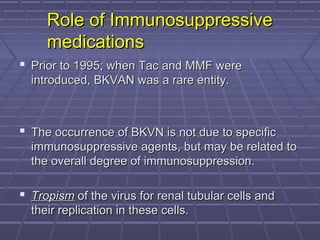
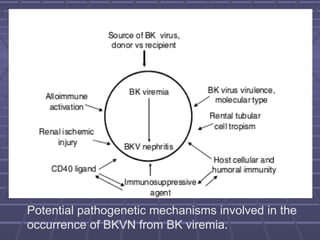
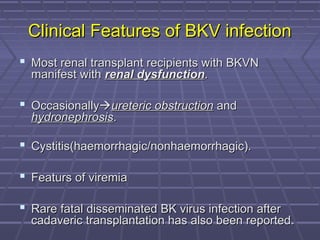
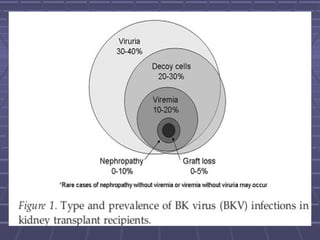
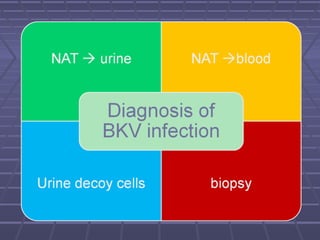
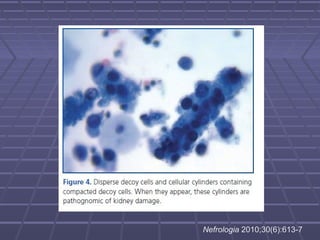
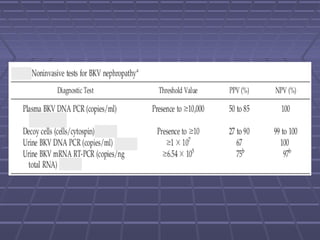
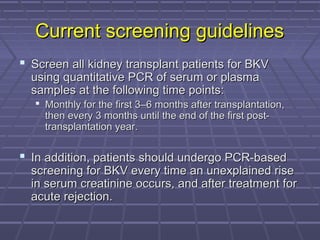
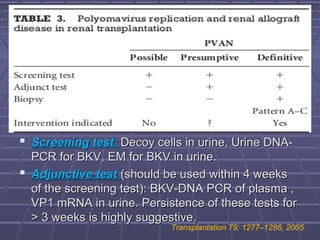
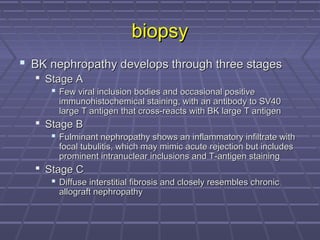
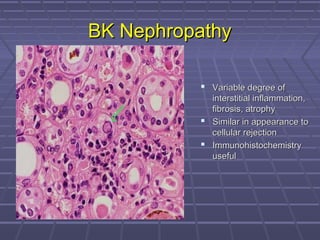
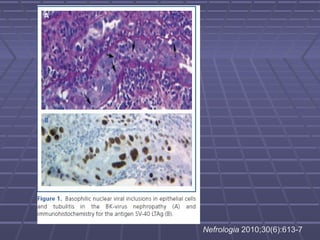
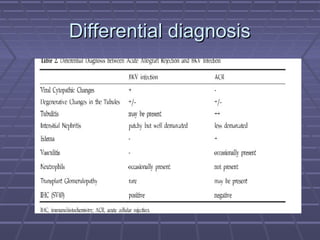
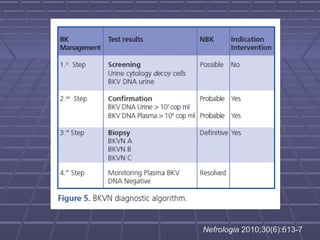
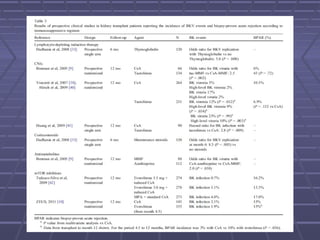
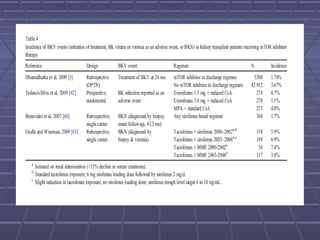
BK virus nephropathy is caused by reactivation of the BK polyomavirus in kidney transplant recipients. It presents as renal dysfunction and can resemble acute rejection on biopsy. Diagnosis involves detecting decoy cells in urine cytology, elevated BK virus DNA in plasma/serum quantitative PCR, and characteristic intranuclear viral inclusions on kidney biopsy. Management primarily focuses on preemptive reduction of immunosuppression when BK viremia is detected to allow immune-mediated viral clearance and prevention of nephropathy. Screening for BK virus by plasma/serum quantitative PCR after transplantation aims to detect reactivation early to guide preemptive immunosuppression adjustments.