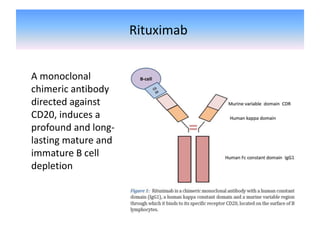
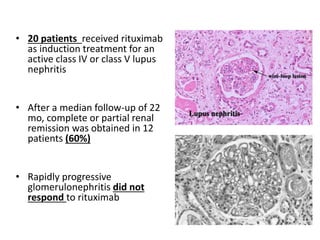
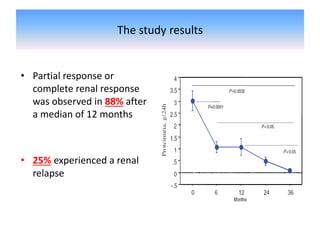
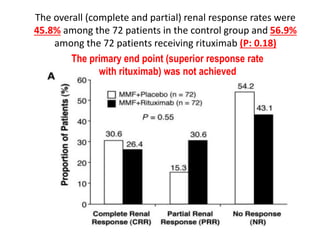
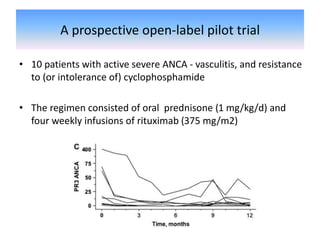
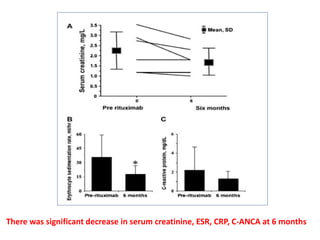
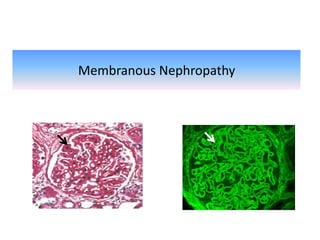
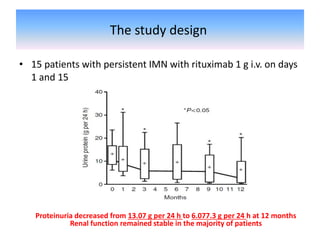
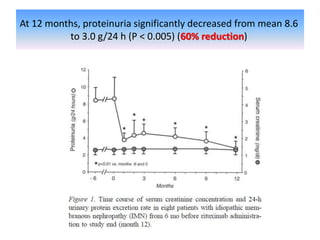
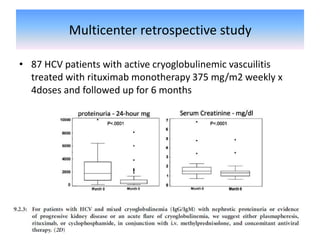
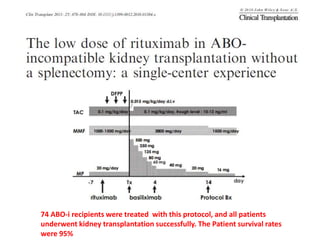
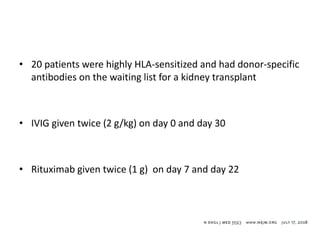
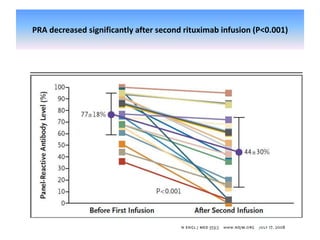
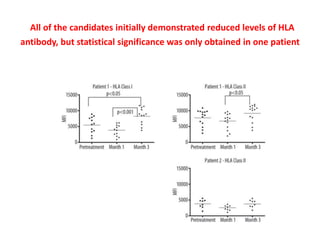
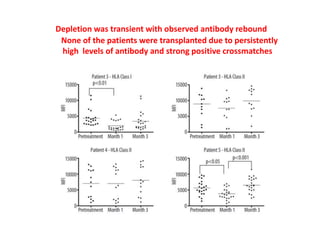
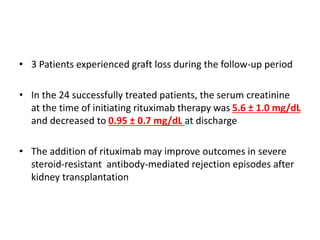
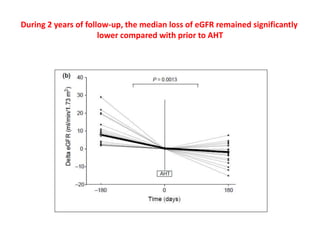
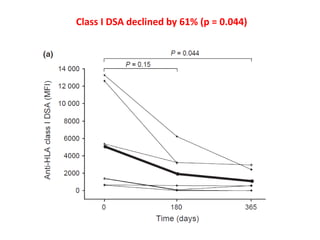
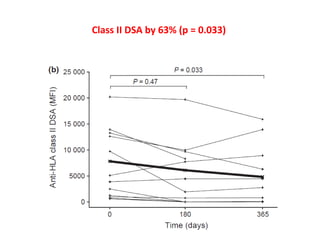
Rituximab is a monoclonal antibody that depletes B cells and has been used to treat various immune-mediated renal diseases and in transplantation. It has shown benefits in refractory lupus nephritis, membranous nephropathy, and cryoglobulinemia in observational studies. However, randomized controlled trials in lupus nephritis did not show a significant benefit of rituximab over standard therapies. Rituximab has also been used successfully in ABO-incompatible and desensitization protocols in renal transplantation to reduce antibody levels, though trials on desensitization showed transient effects. Rituximab may improve outcomes for severe antibody-mediated rejection. Further large randomized trials are still