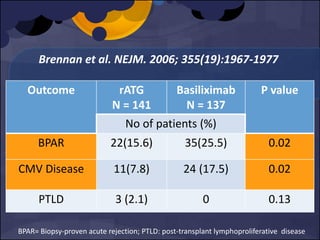
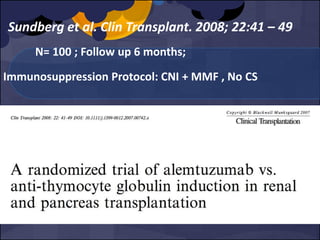
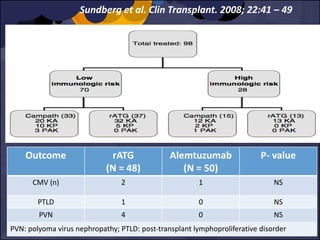
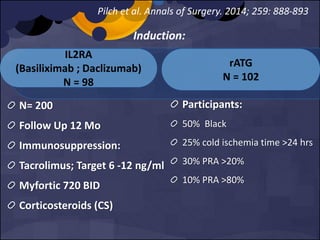
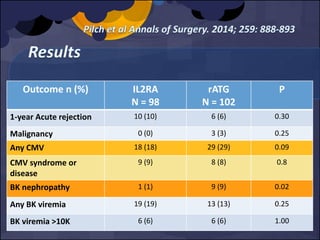
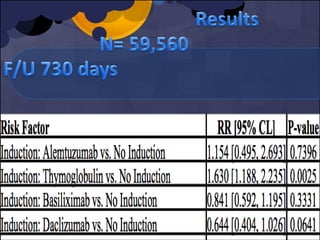
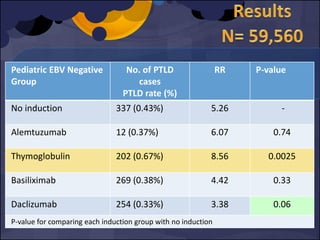
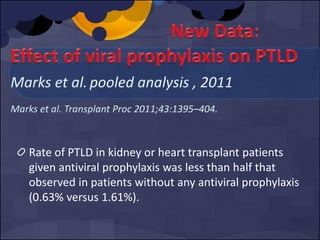
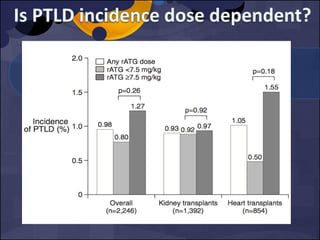
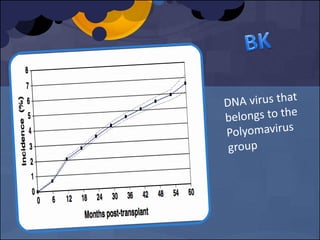
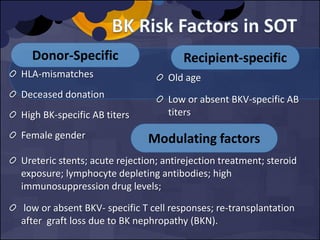
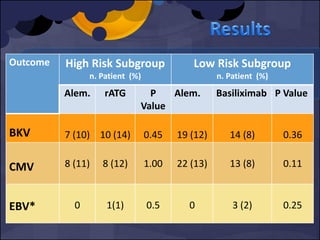
The document summarizes various studies comparing the risk of viral infections like CMV, BK virus, EBV with different induction therapies post-transplant like rATG, IL-2 receptor inhibitors, alemtuzumab. While some studies found higher rates of CMV with rATG, the evidence is inconsistent. The risk of BK virus seems higher with rATG in one recent RCT but not in pooled analyses. PTLD risk may be higher with rATG but the evidence is also inconsistent. Overall the personalized approach is needed to balance rejection risk versus infection risk for each patient.



![CMV donor +, recipient – [D+R–]
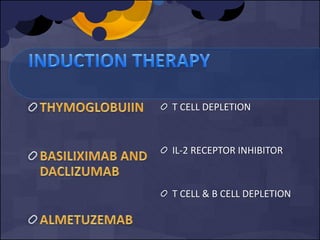
Use of lymphocyte-depleting agents
Immunosuppressive protocol ( type of drug, dose, timing,
duration)
Transplant Recipient factors (e.g. age, comorbidity,
leukopenia and lymphopenia, genetic factors, cold
ischemia time, critical illness, stress)
Transplant type. E.g. Lung and small intestinal
Confections with human herpes virus (HHV)-6 and HHV-7](https://image.slidesharecdn.com/e5db3dcb-0f2e-4c18-b509-35fc5620ce36-160206165424/85/FINAL_INDUCTION_VIRAL-INF_PRESENTATION-7-320.jpg)
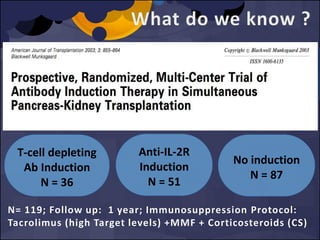








![MA of 10 RCTs, N = 1223 patients
Morgan et al. Transplantation 2012; 93: 1179 -1188
[6 RCT]
Only One Study showed Sig
higher incidence of CMV
infection with Alemtuzumab
vs no induction (28% vs 12%,
p= 0.03)
No tissue invasive episodes
in alemtuzumab.](https://image.slidesharecdn.com/e5db3dcb-0f2e-4c18-b509-35fc5620ce36-160206165424/85/FINAL_INDUCTION_VIRAL-INF_PRESENTATION-36-320.jpg)
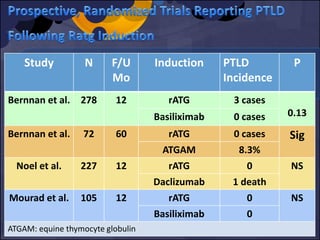
![6 RCT
4 RCT No PTLD cases in alemtuzumab or control gps.
[max. F/U 5 years]
1 RCT 1 PTLD case in alemtuzumab gp vs. none in
control gp. [F/U 36 Mo]
1 RCT Two PTLD cases in alemtuzumab gp vs. one
in basiliximab. [ F/U 12 Mo]
MA of 10 RCTs, N = 1223 patients
Morgan et al. Transplantation 2012; 93: 1179 -1188](https://image.slidesharecdn.com/e5db3dcb-0f2e-4c18-b509-35fc5620ce36-160206165424/85/FINAL_INDUCTION_VIRAL-INF_PRESENTATION-37-320.jpg)




