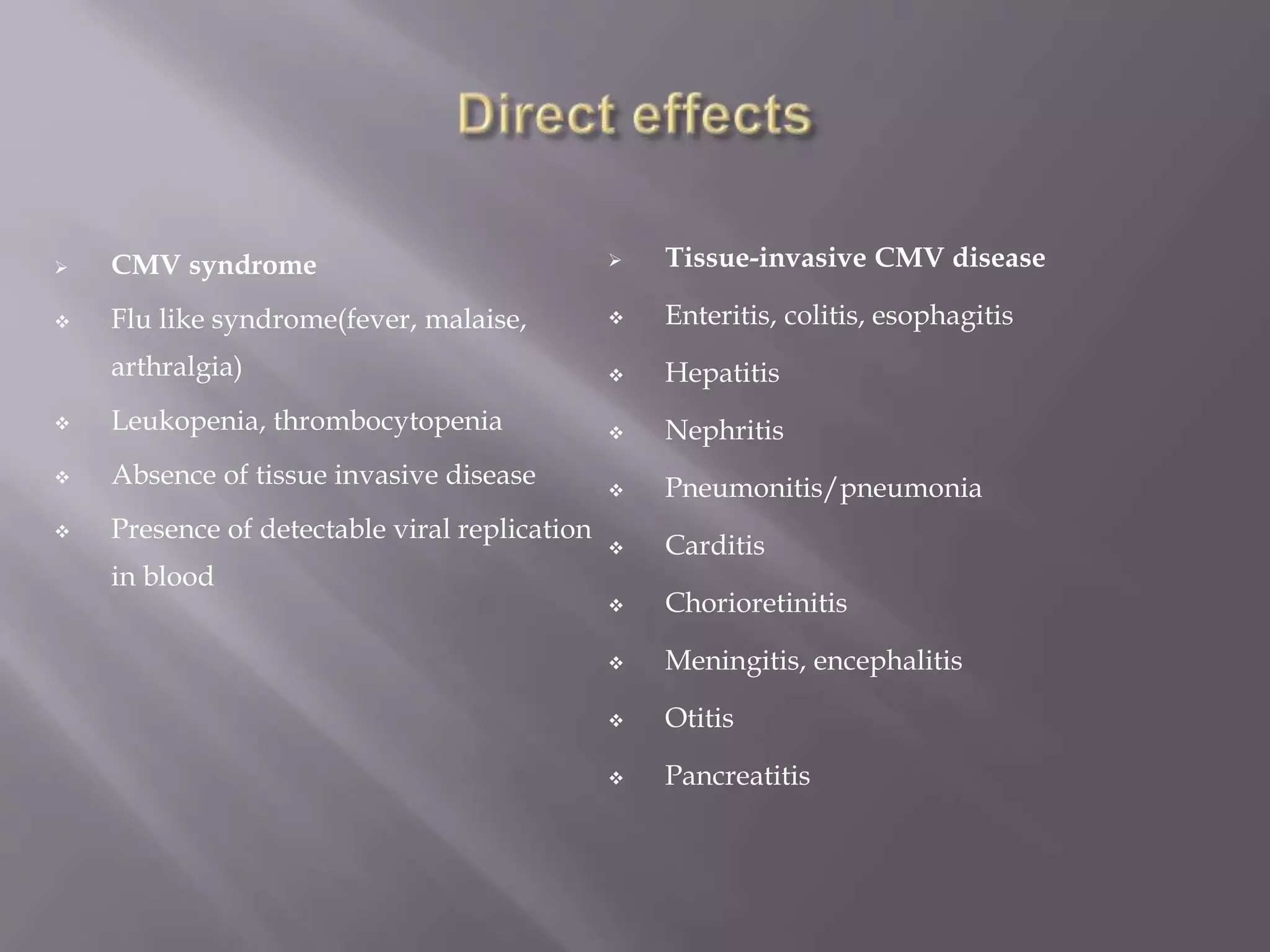
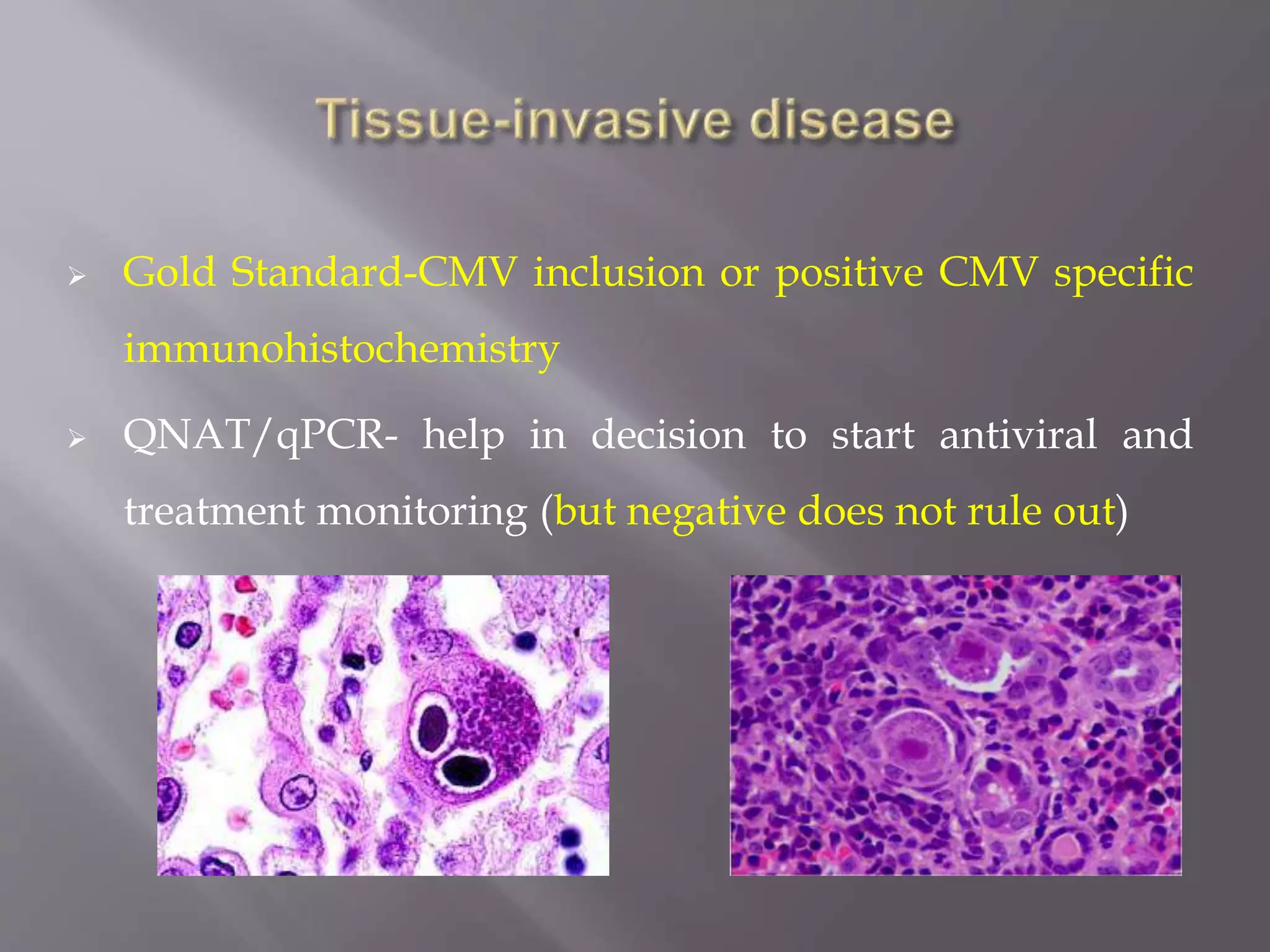
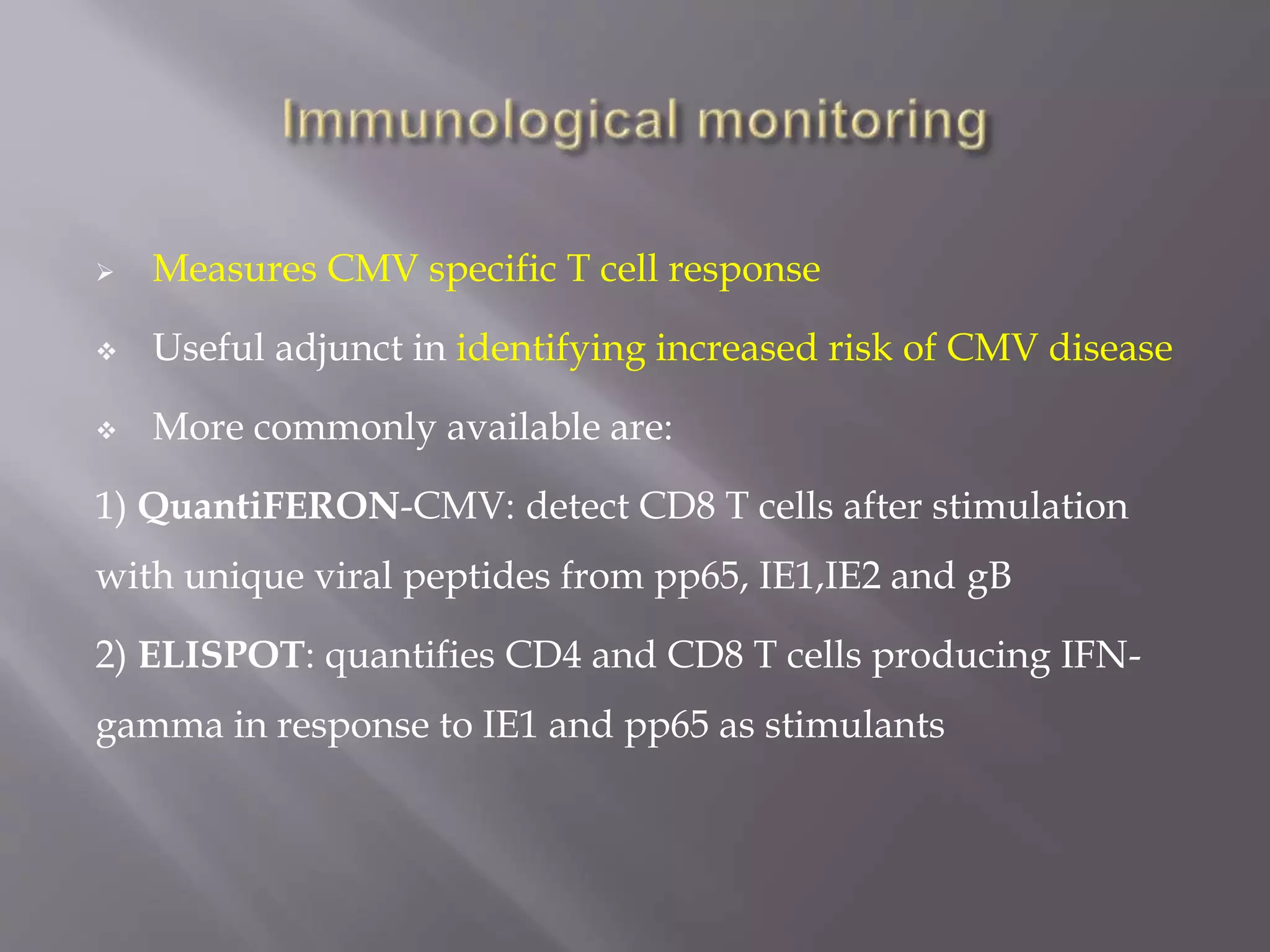
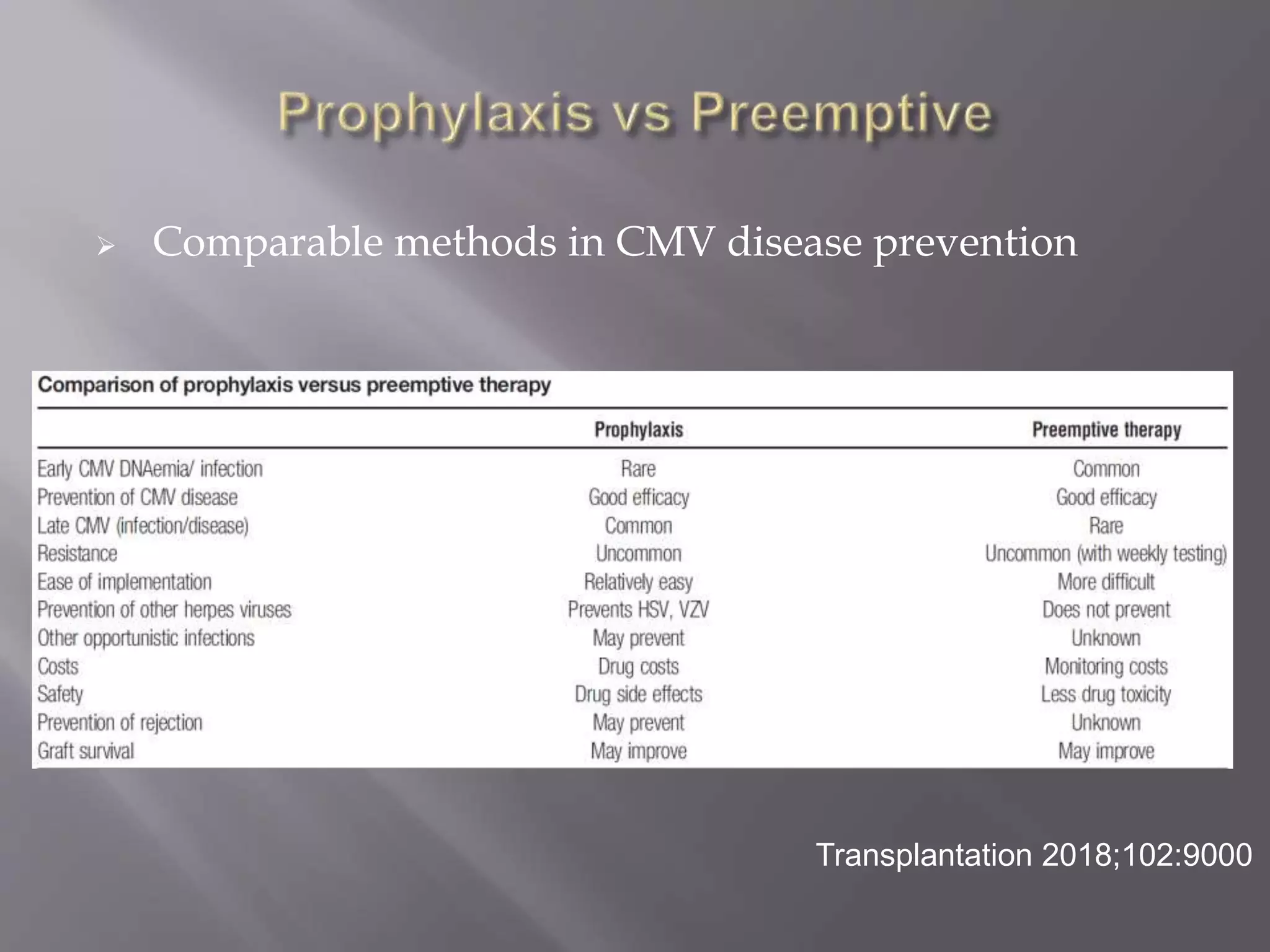
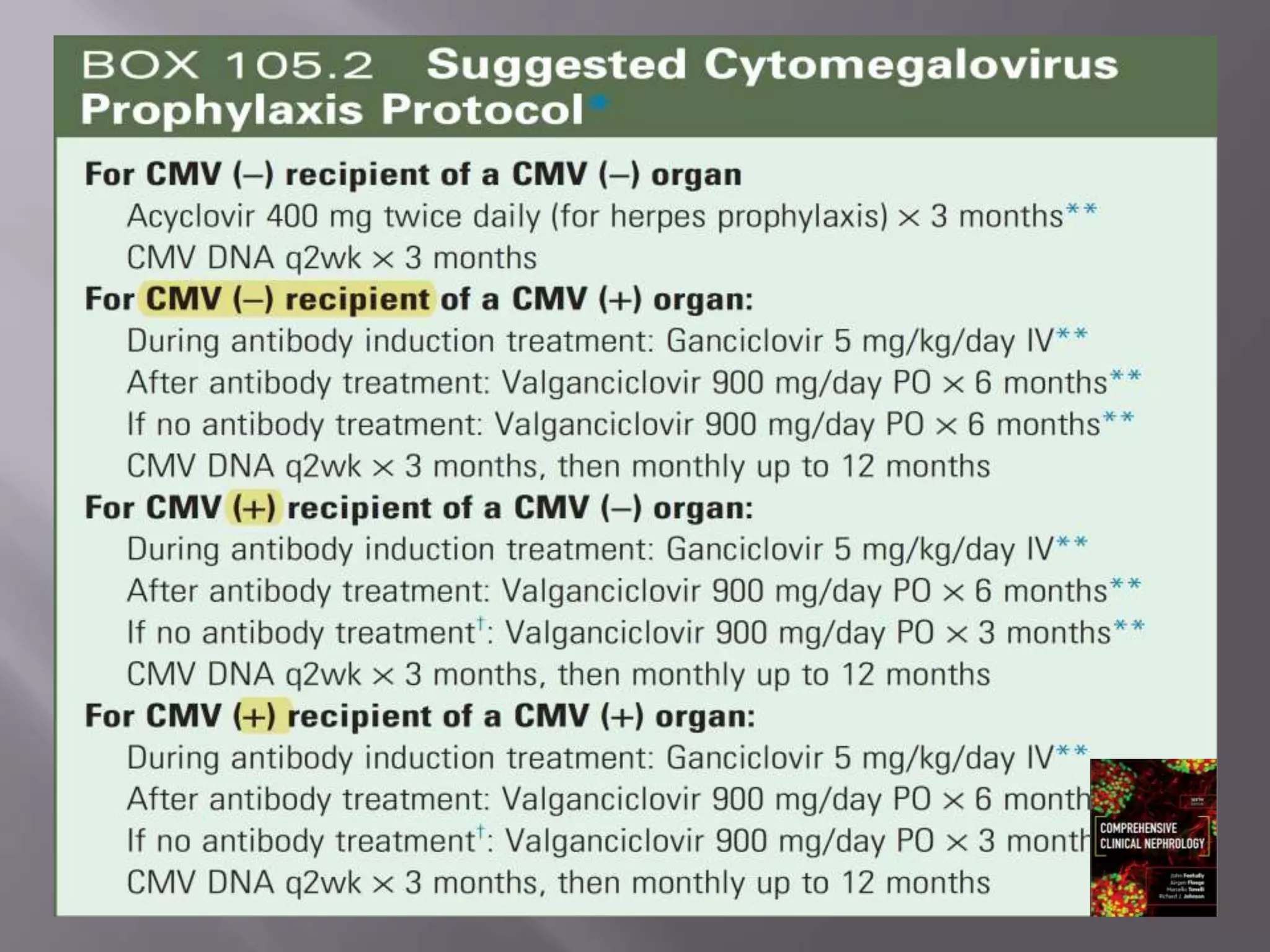
This document discusses cytomegalovirus (CMV) infection in renal transplant patients. It begins with an introduction and overview of CMV, including its definition, epidemiology, risk factors, pathogenesis, clinical presentation, diagnosis, prevention and treatment. It then discusses CMV in more depth, covering topics like transmission, replication within host cells, immune evasion mechanisms, clinical manifestations in different organs, diagnosis methods, prevention strategies like prophylaxis and preemptive therapy, antiviral treatment and drug resistance.