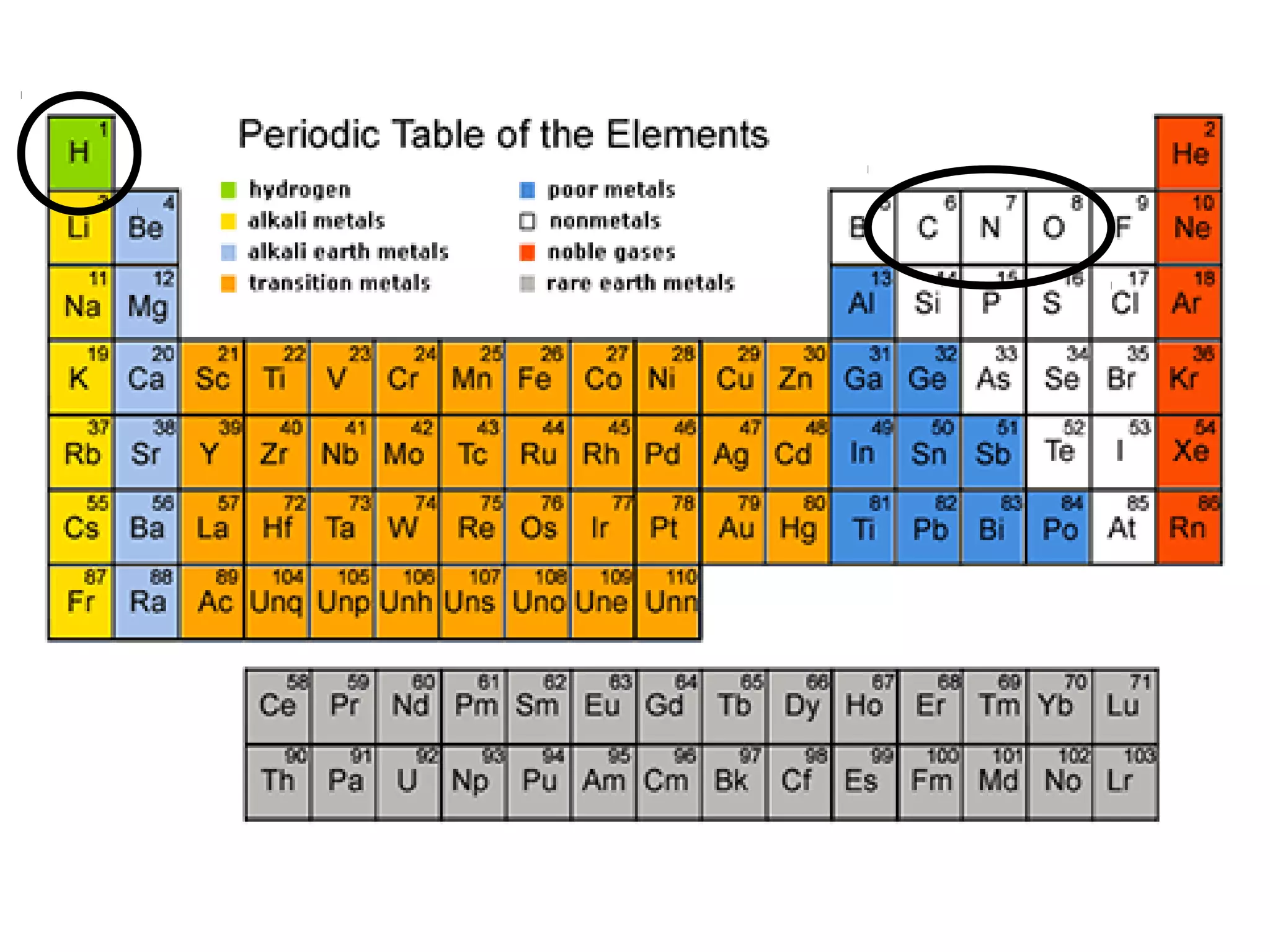
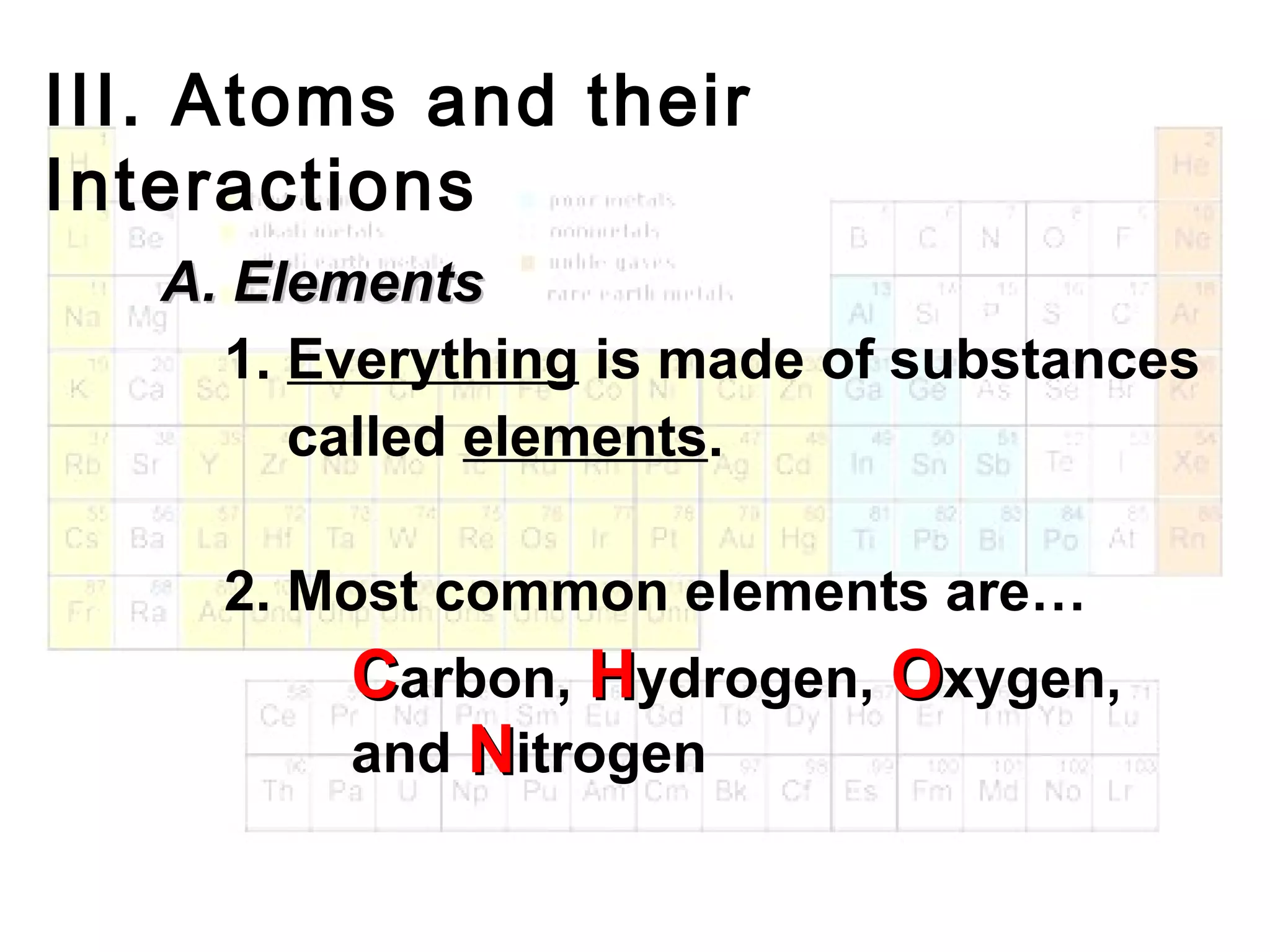
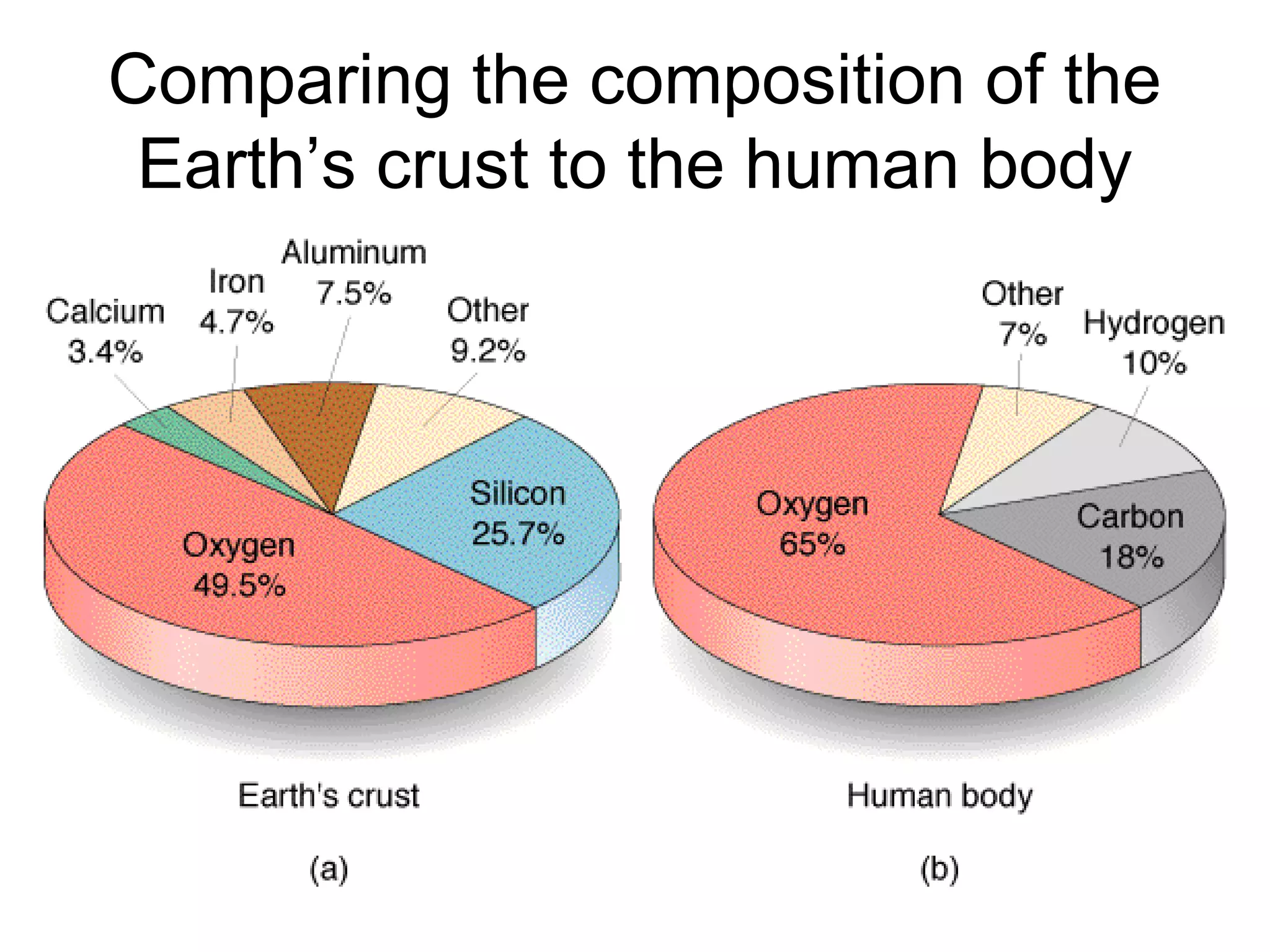
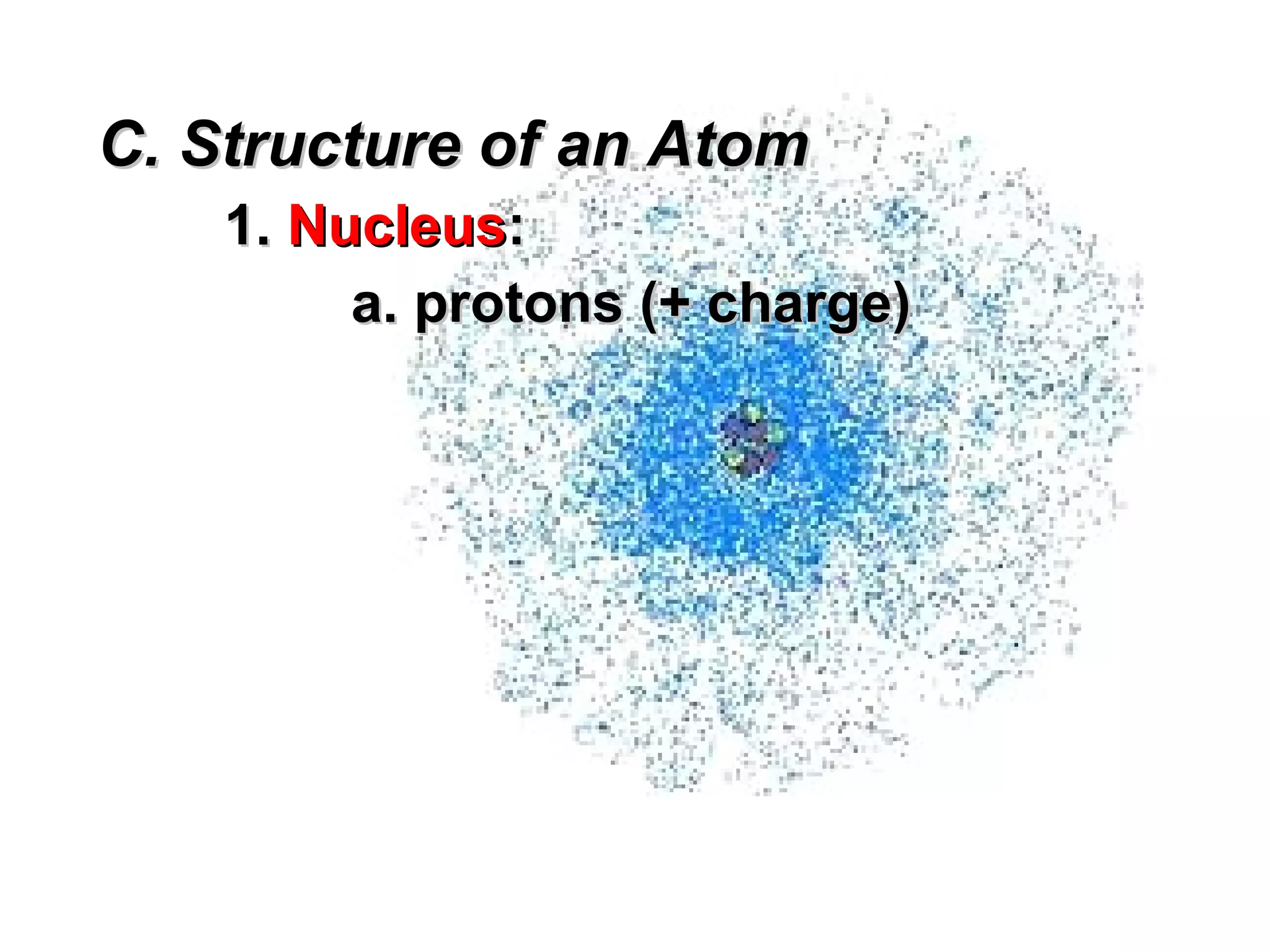
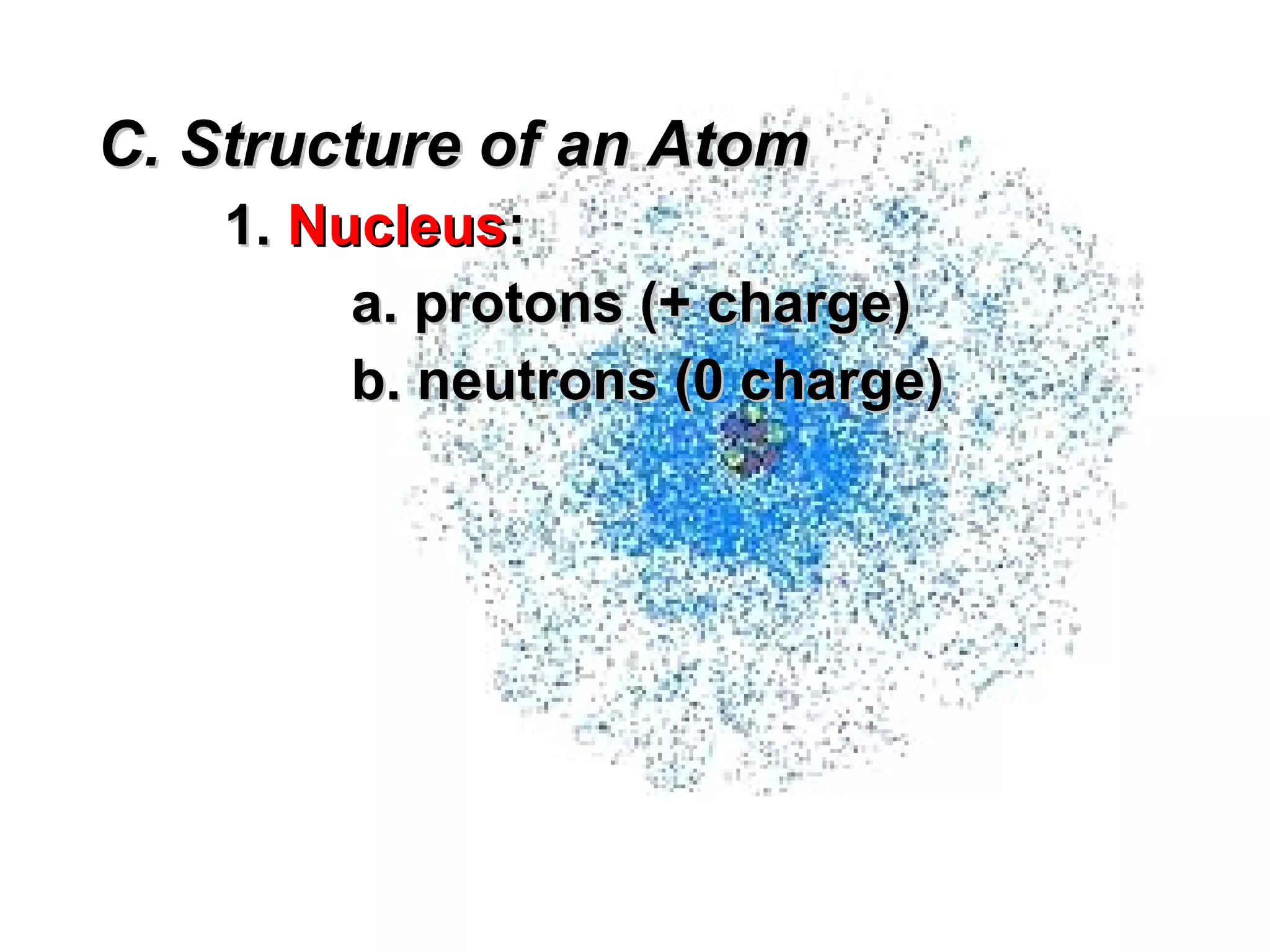
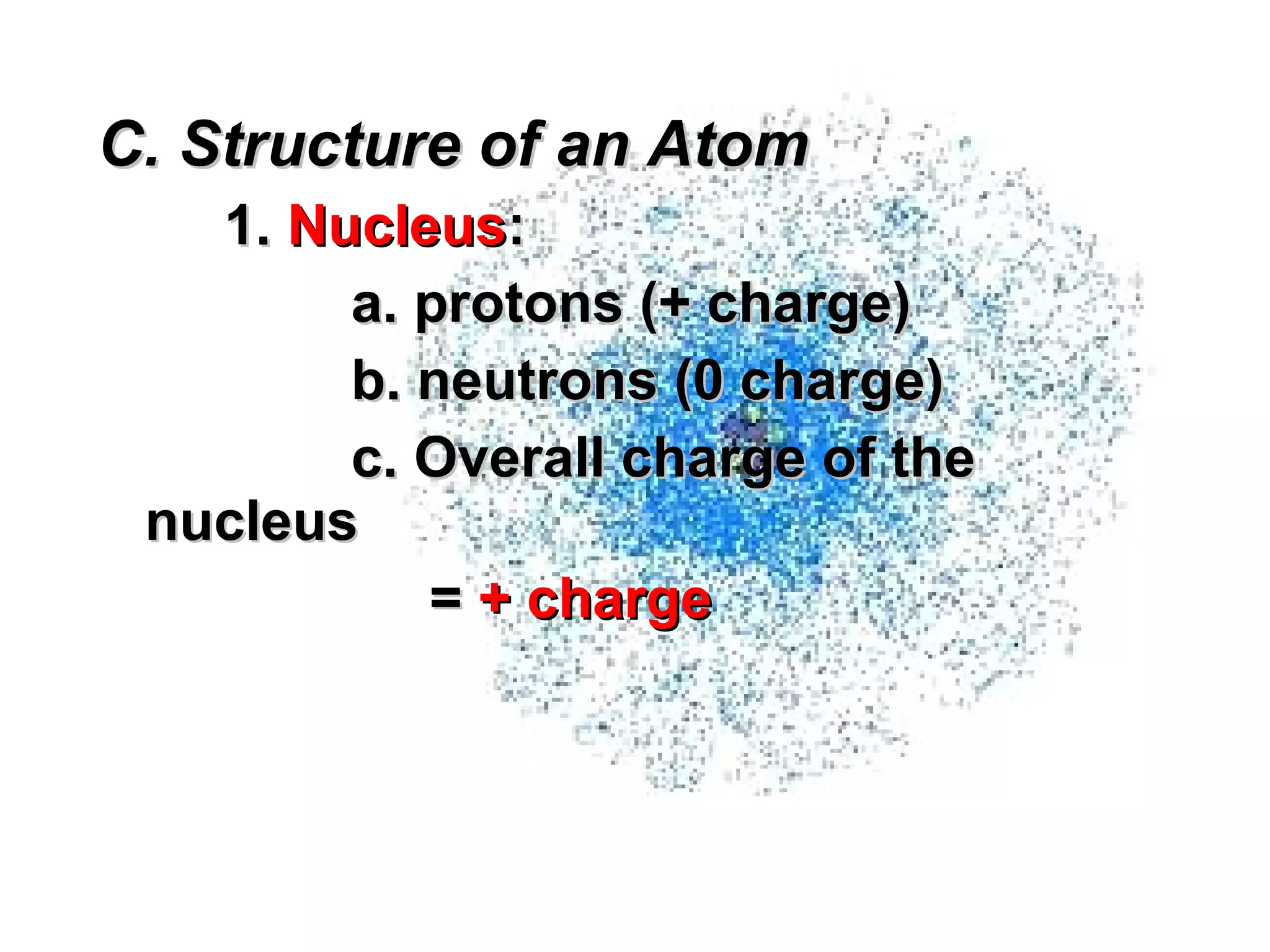
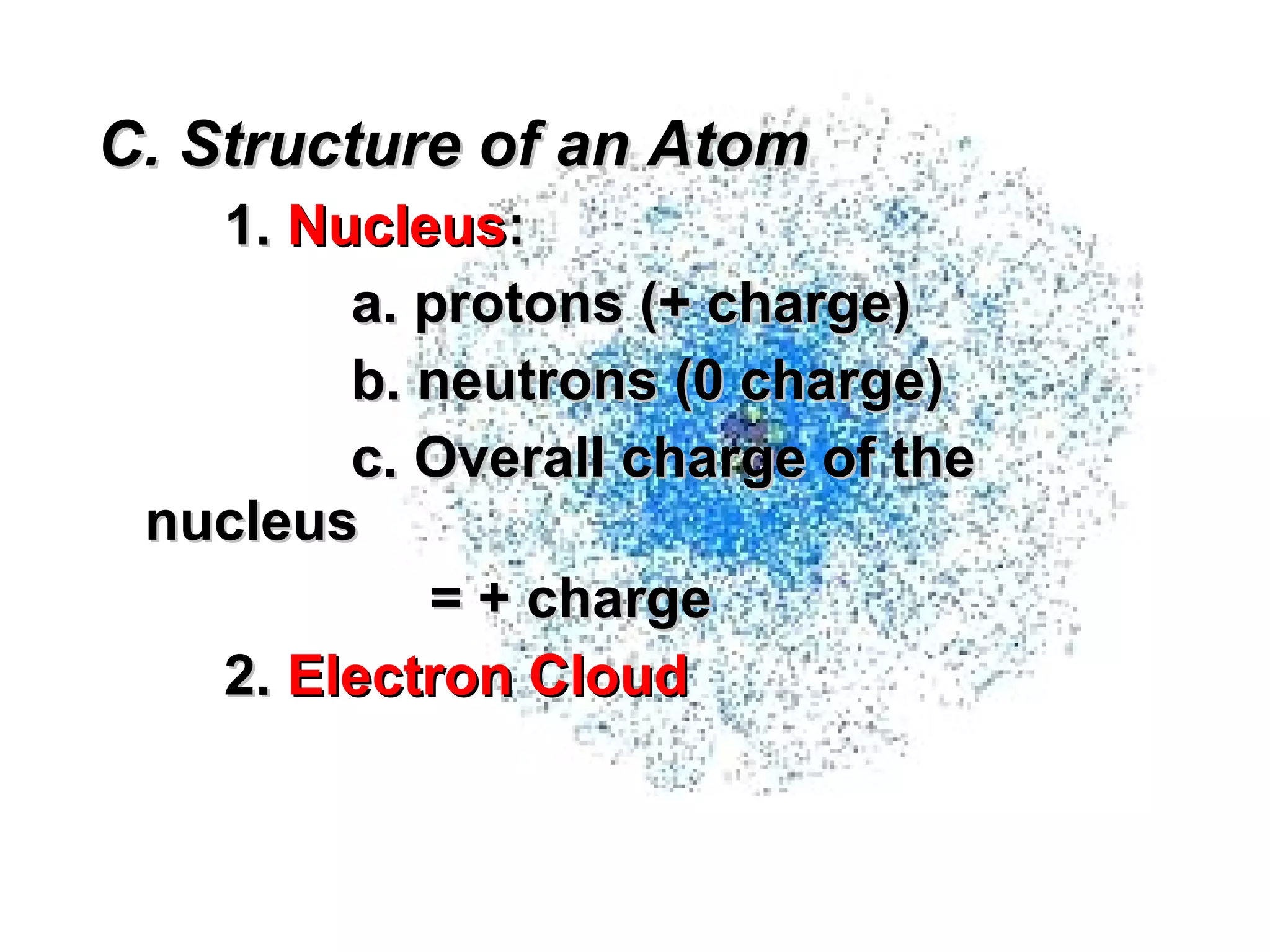
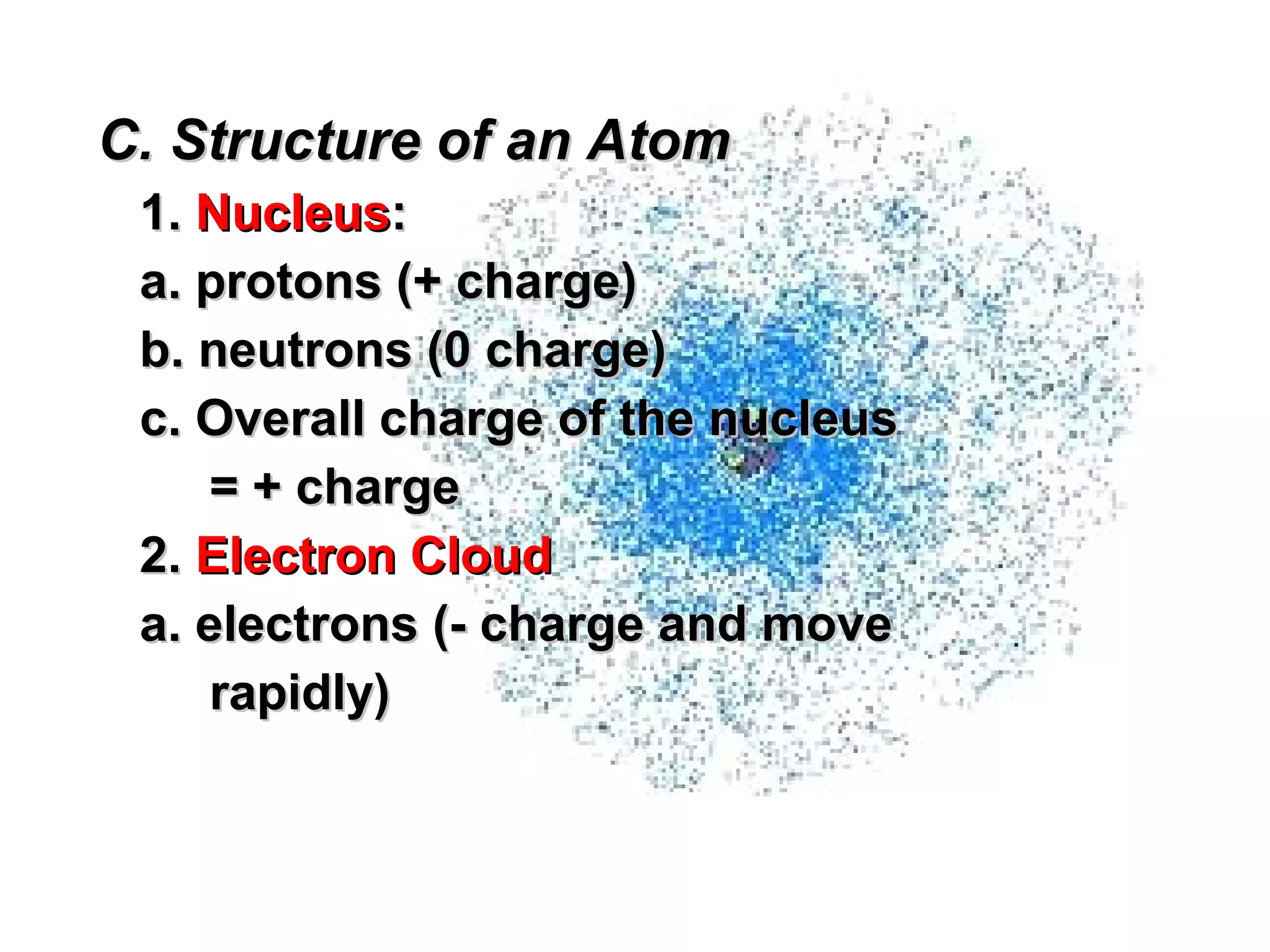
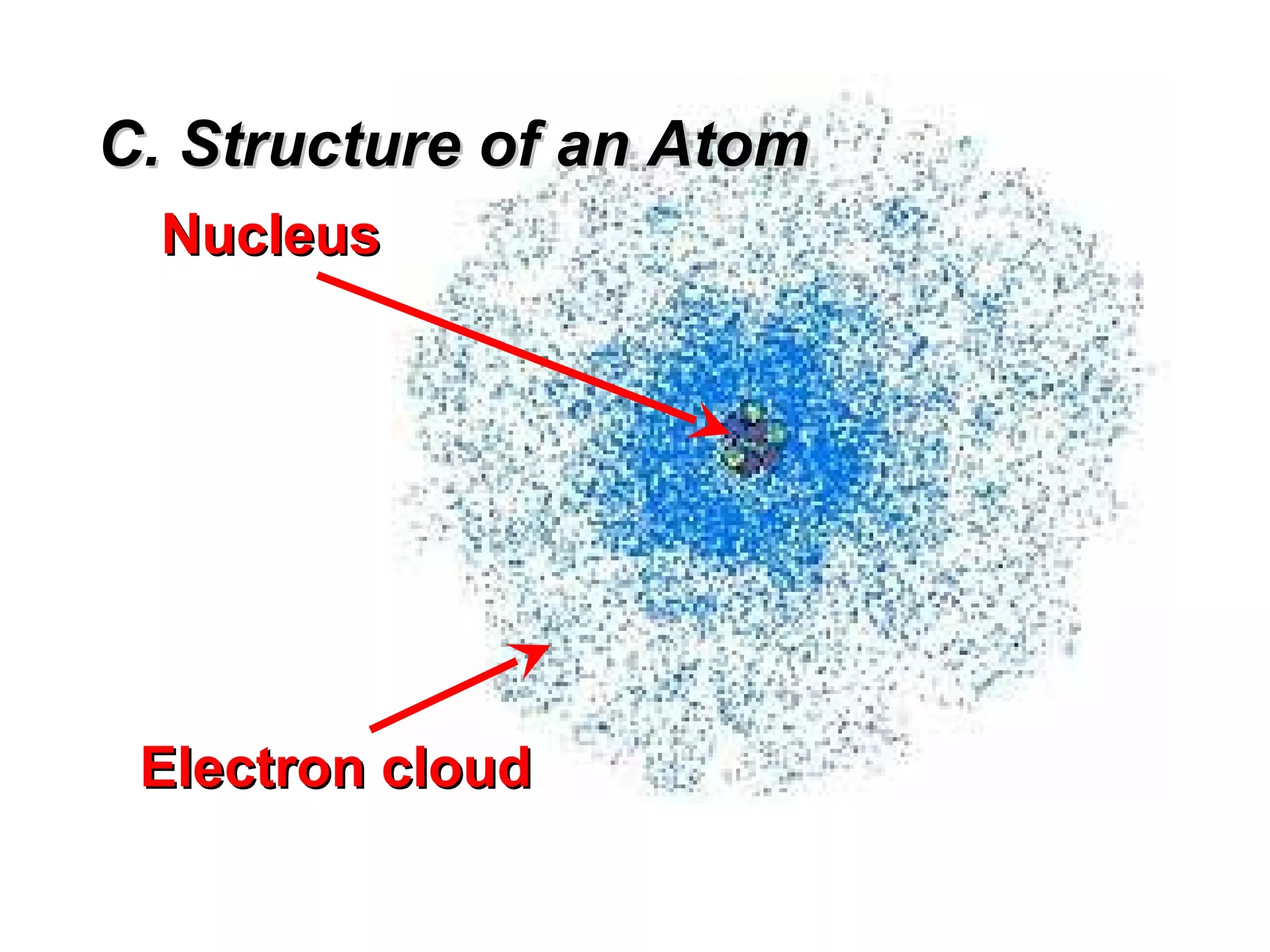
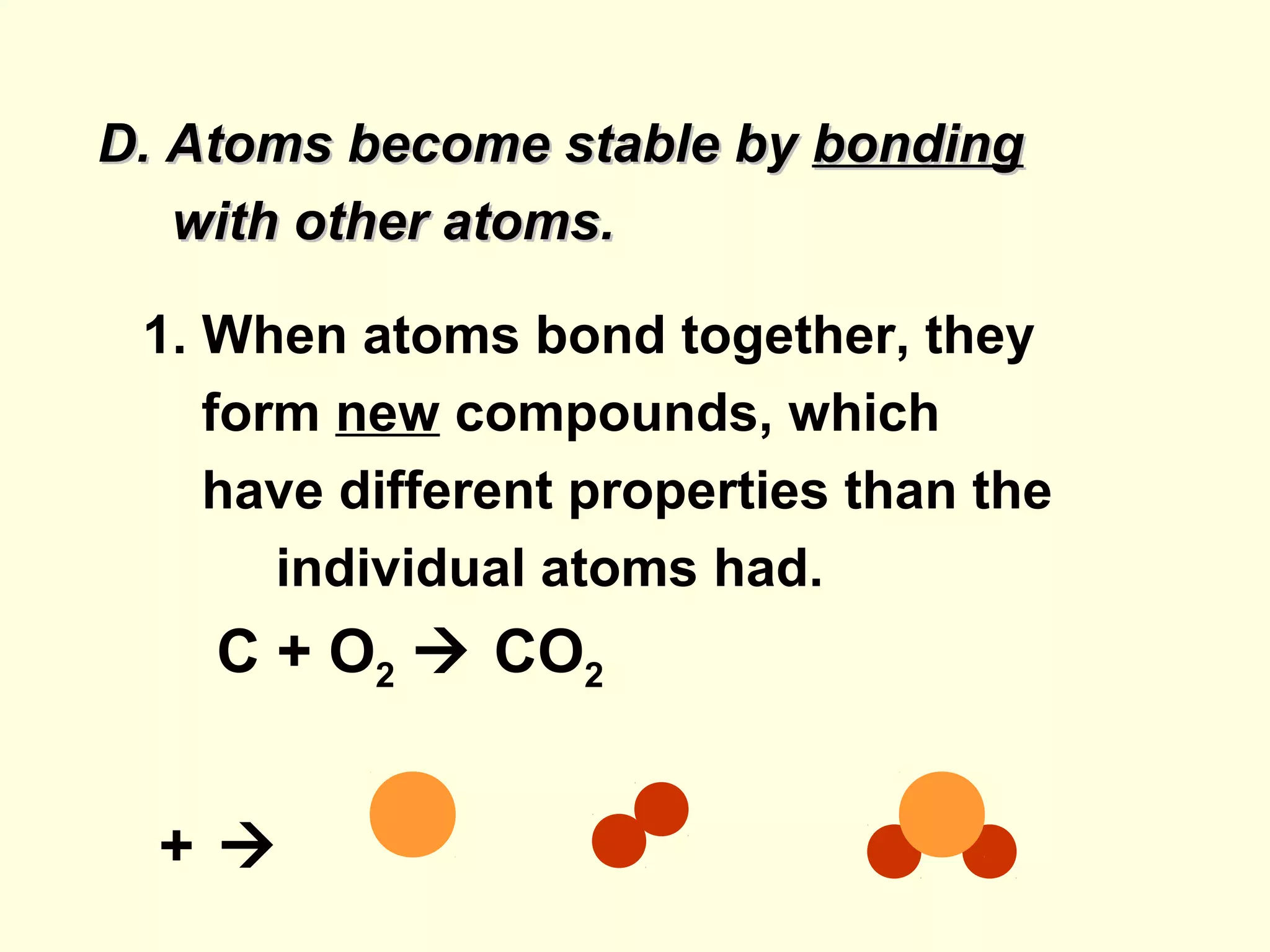
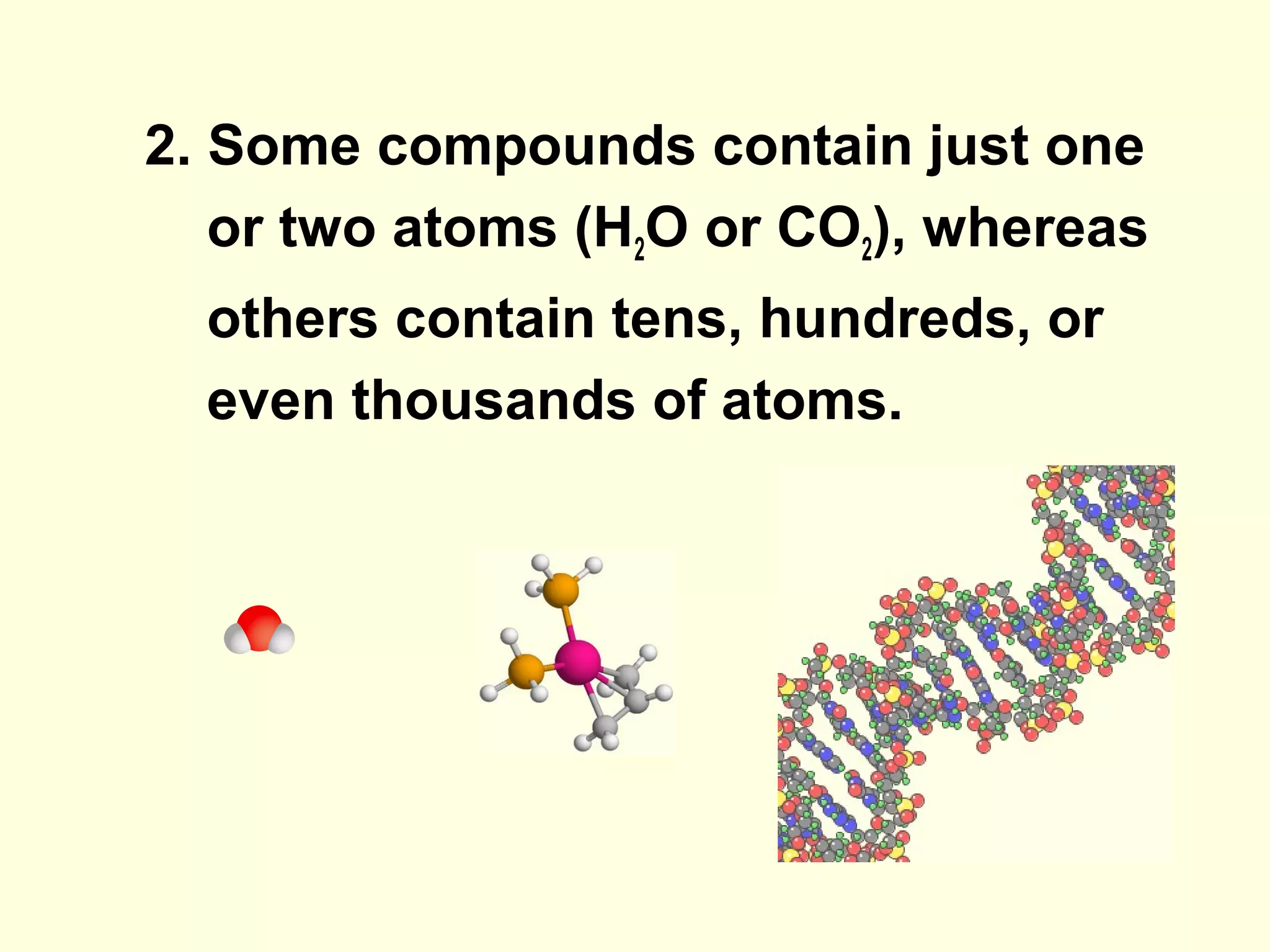
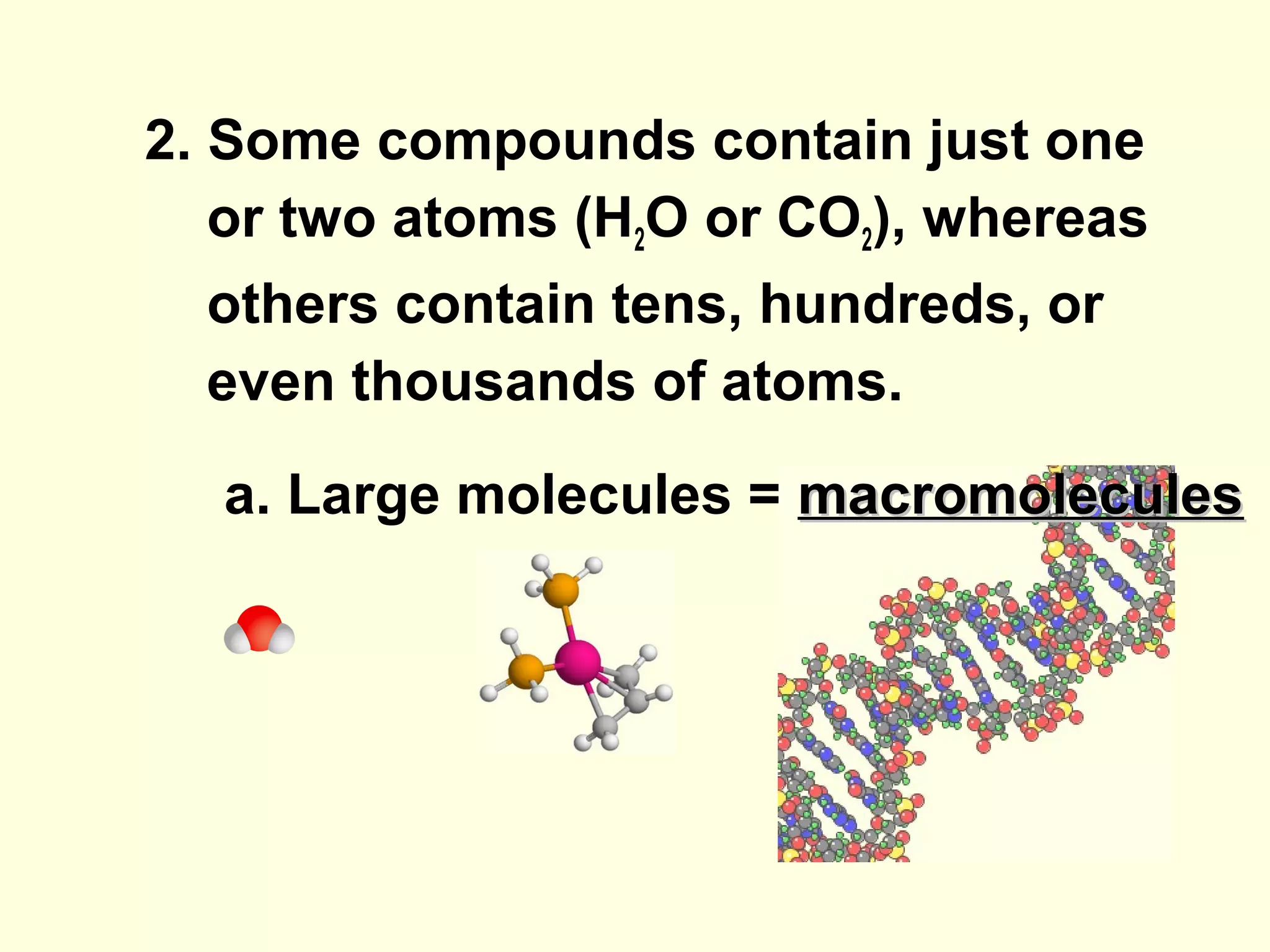
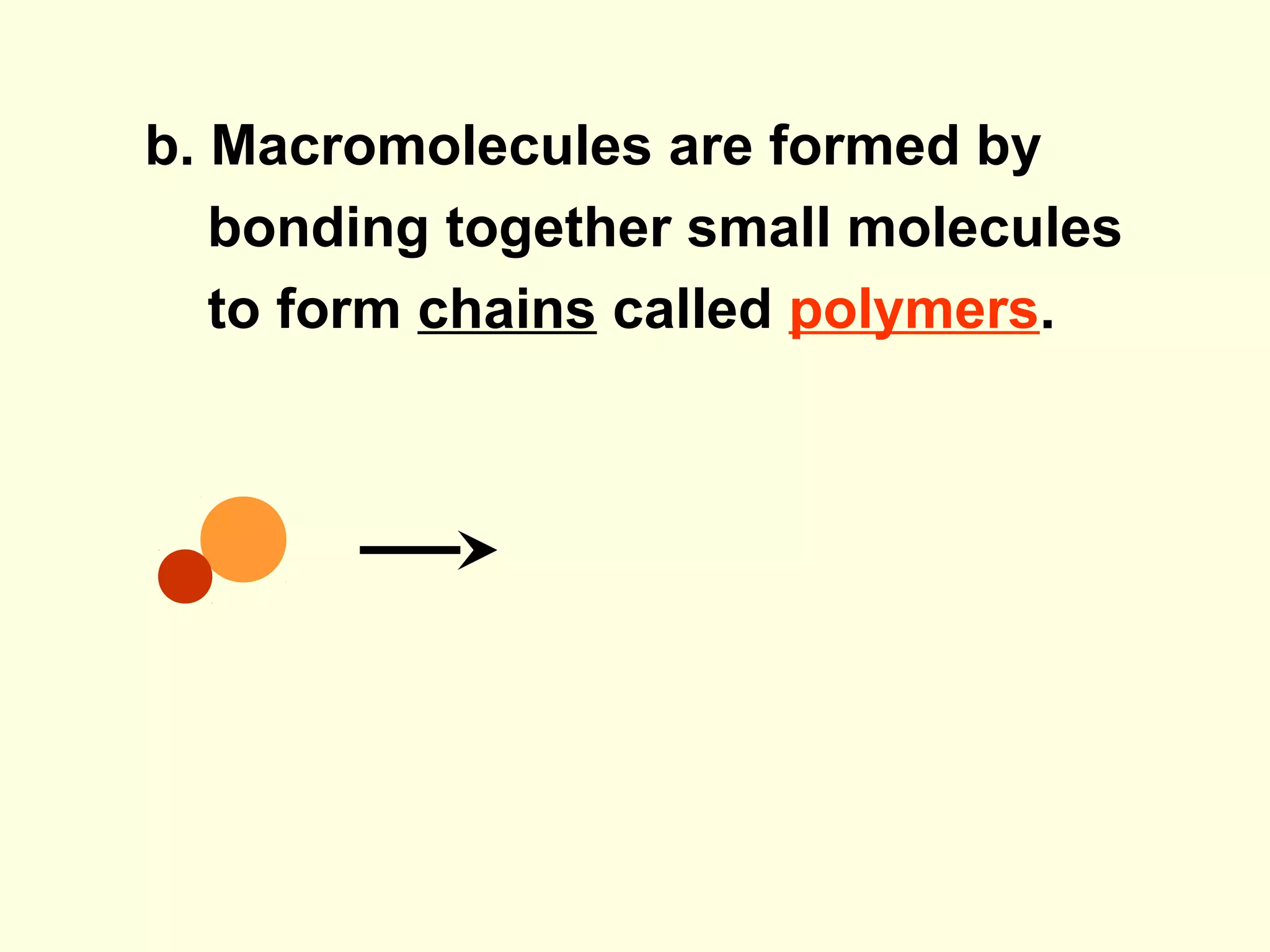
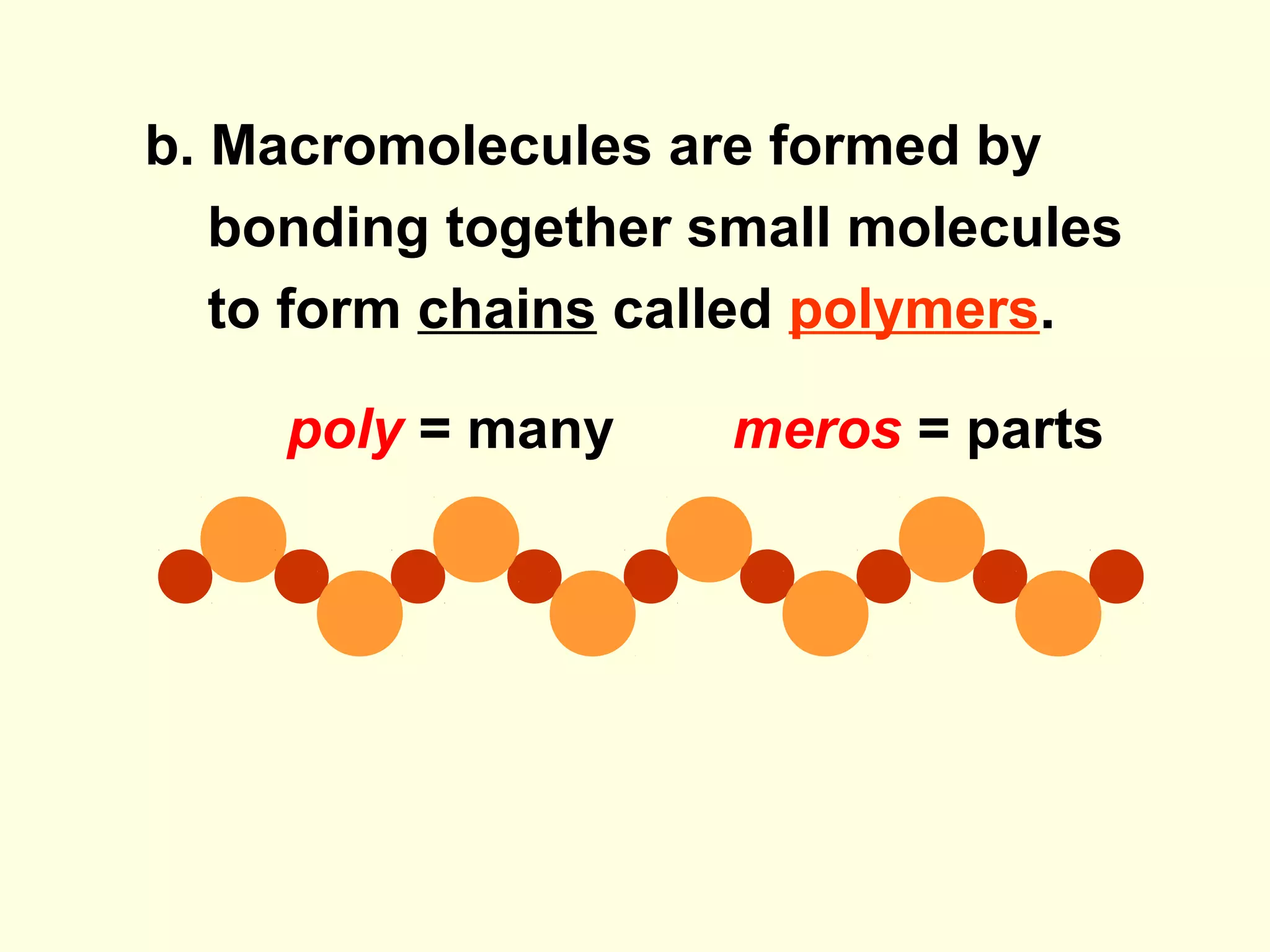
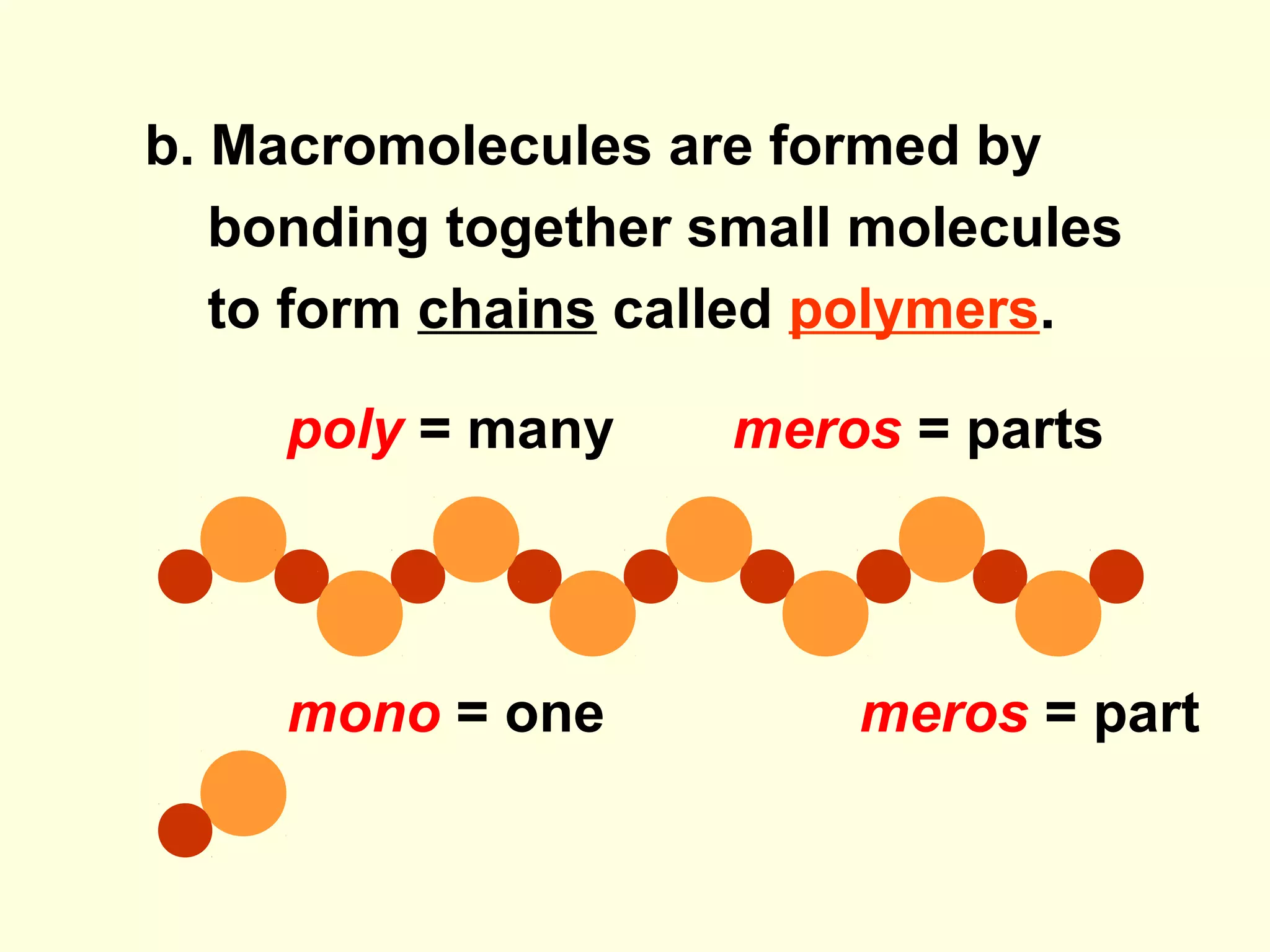
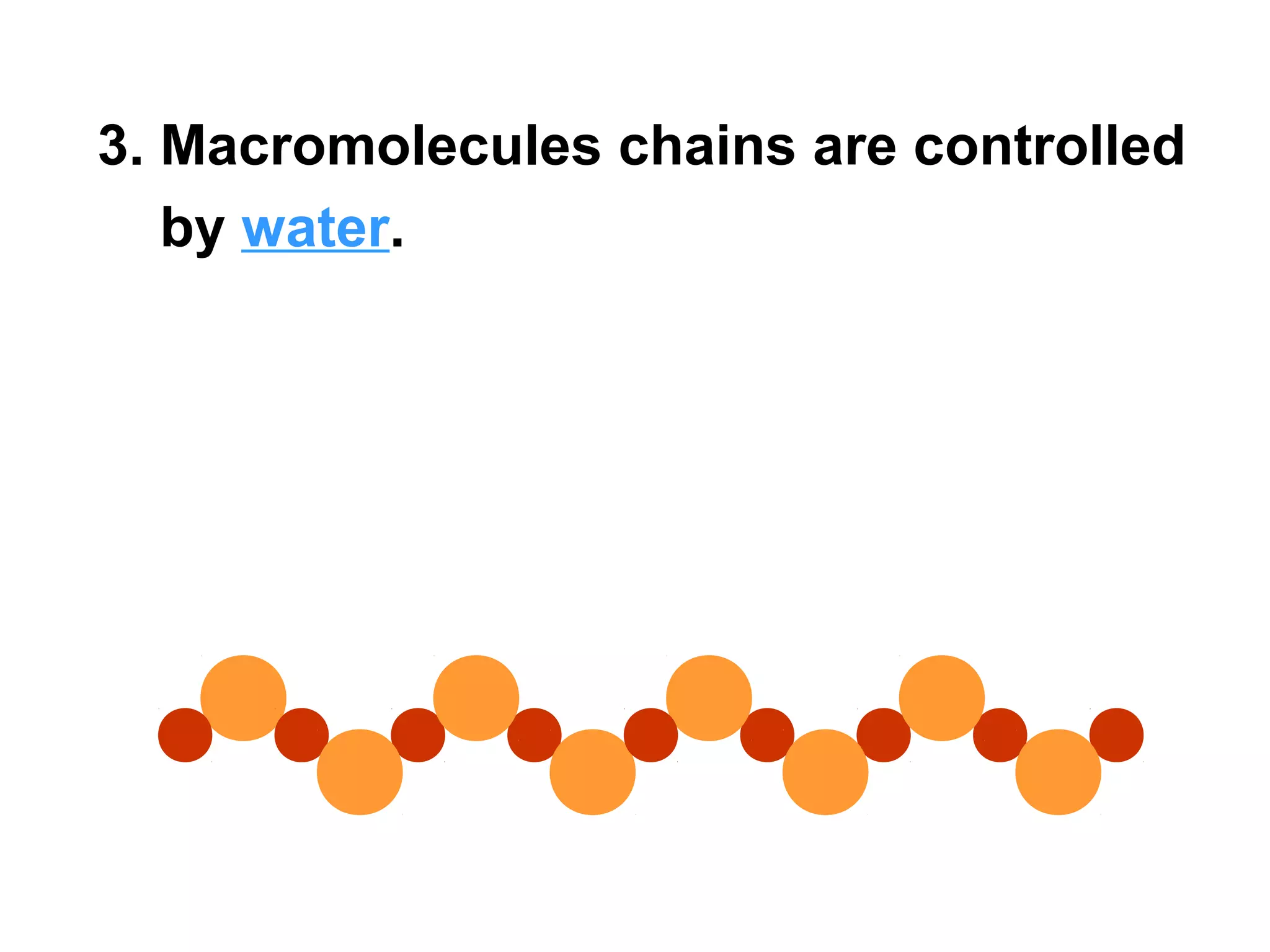
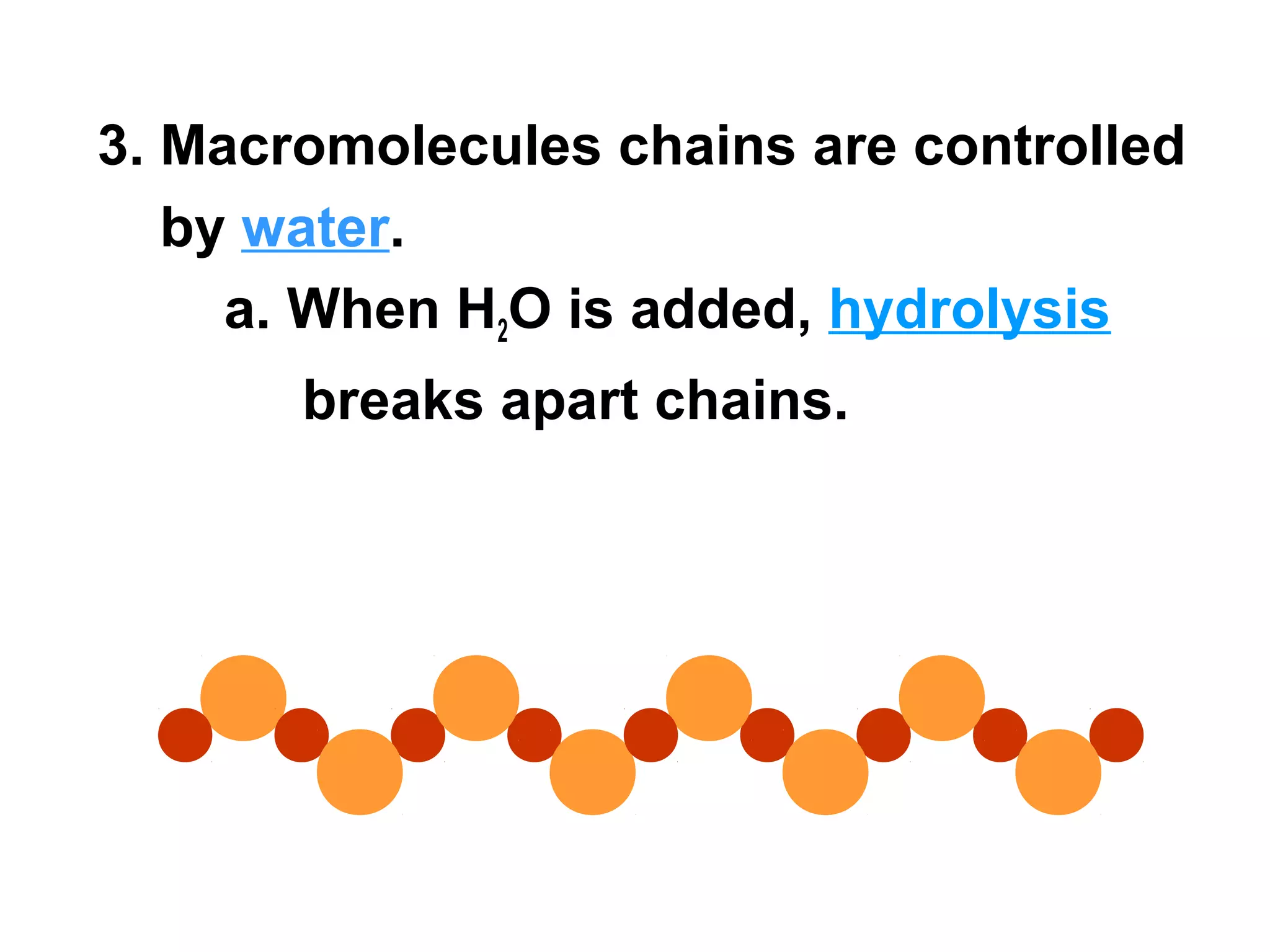
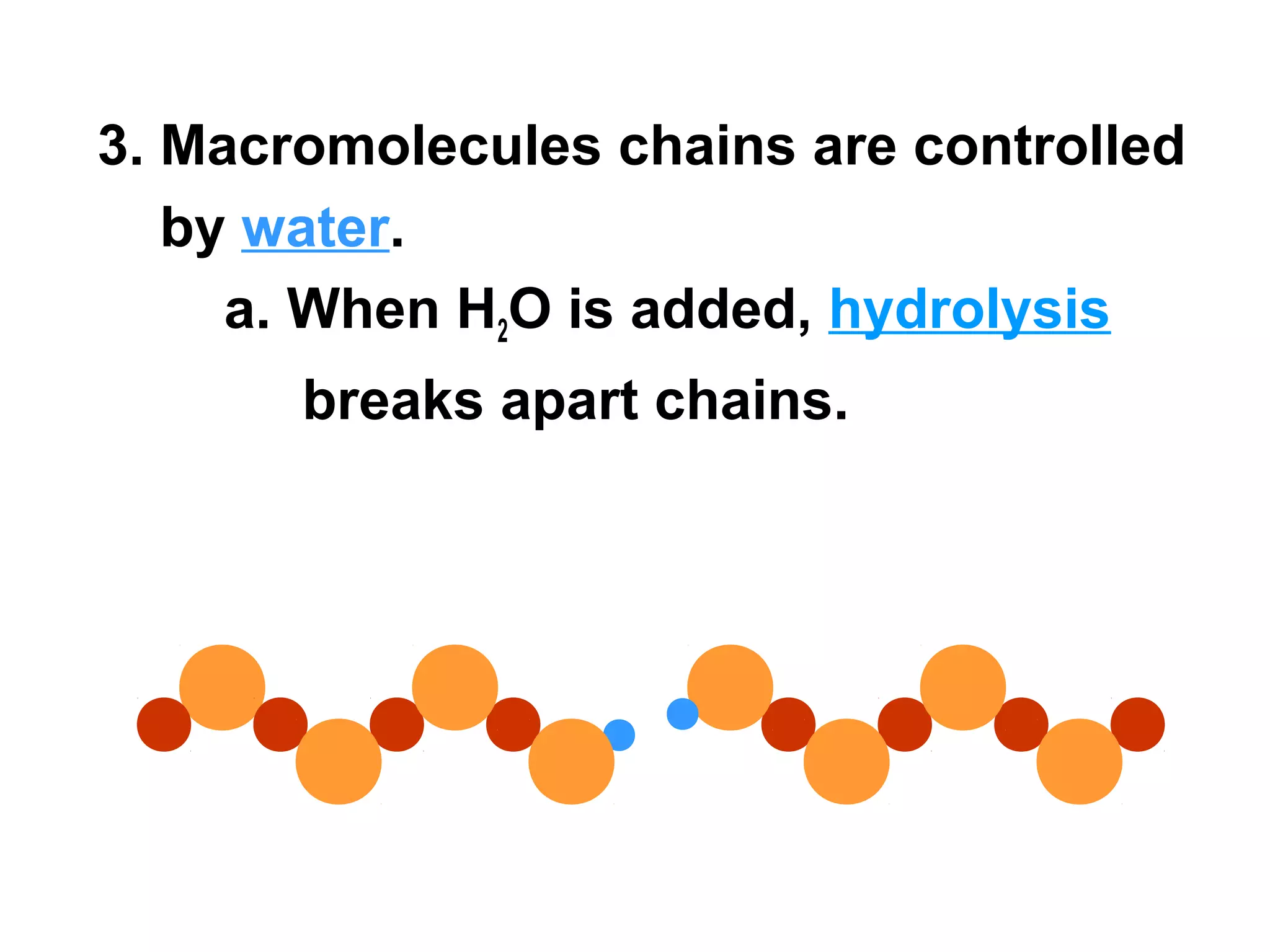
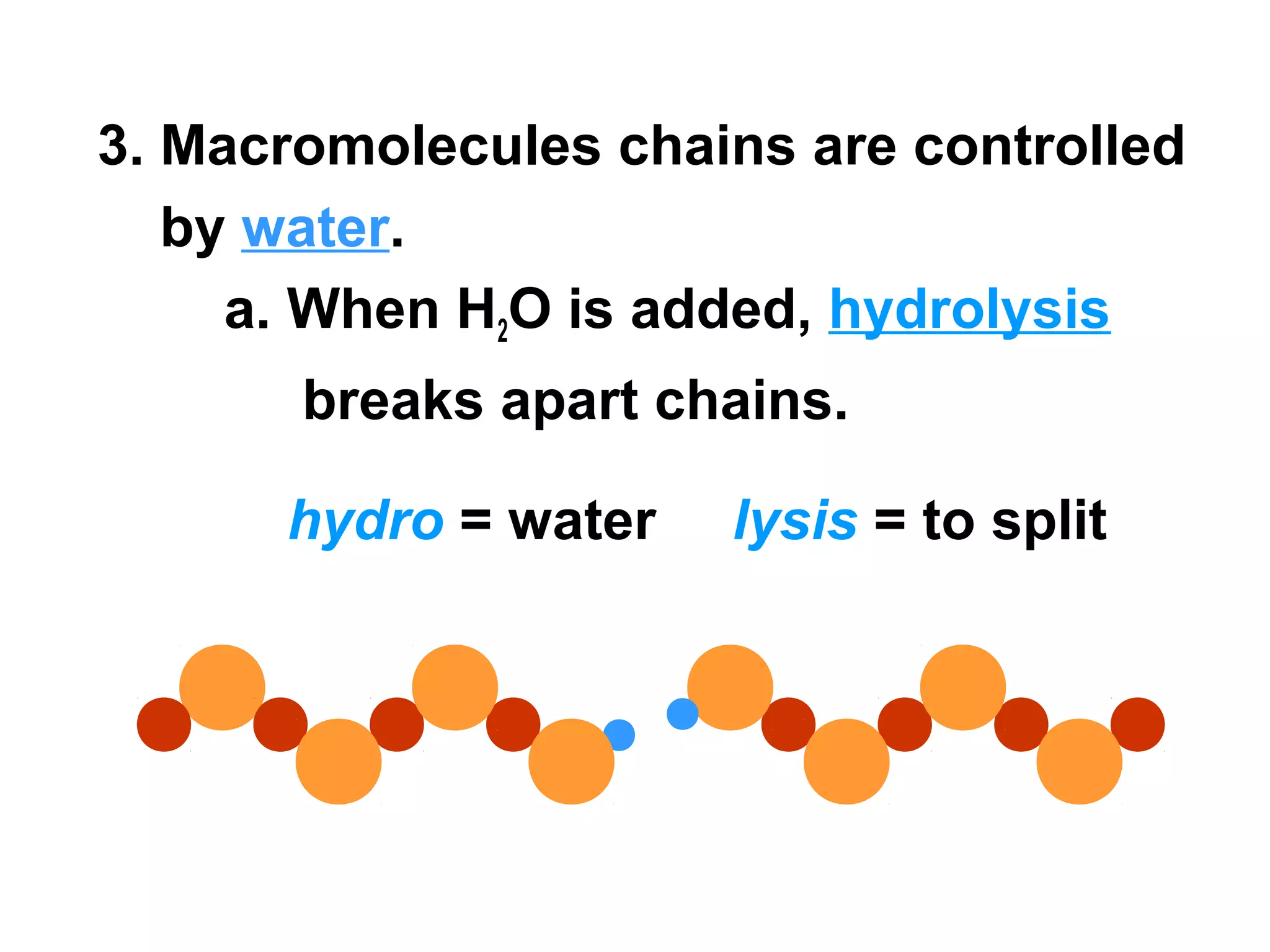
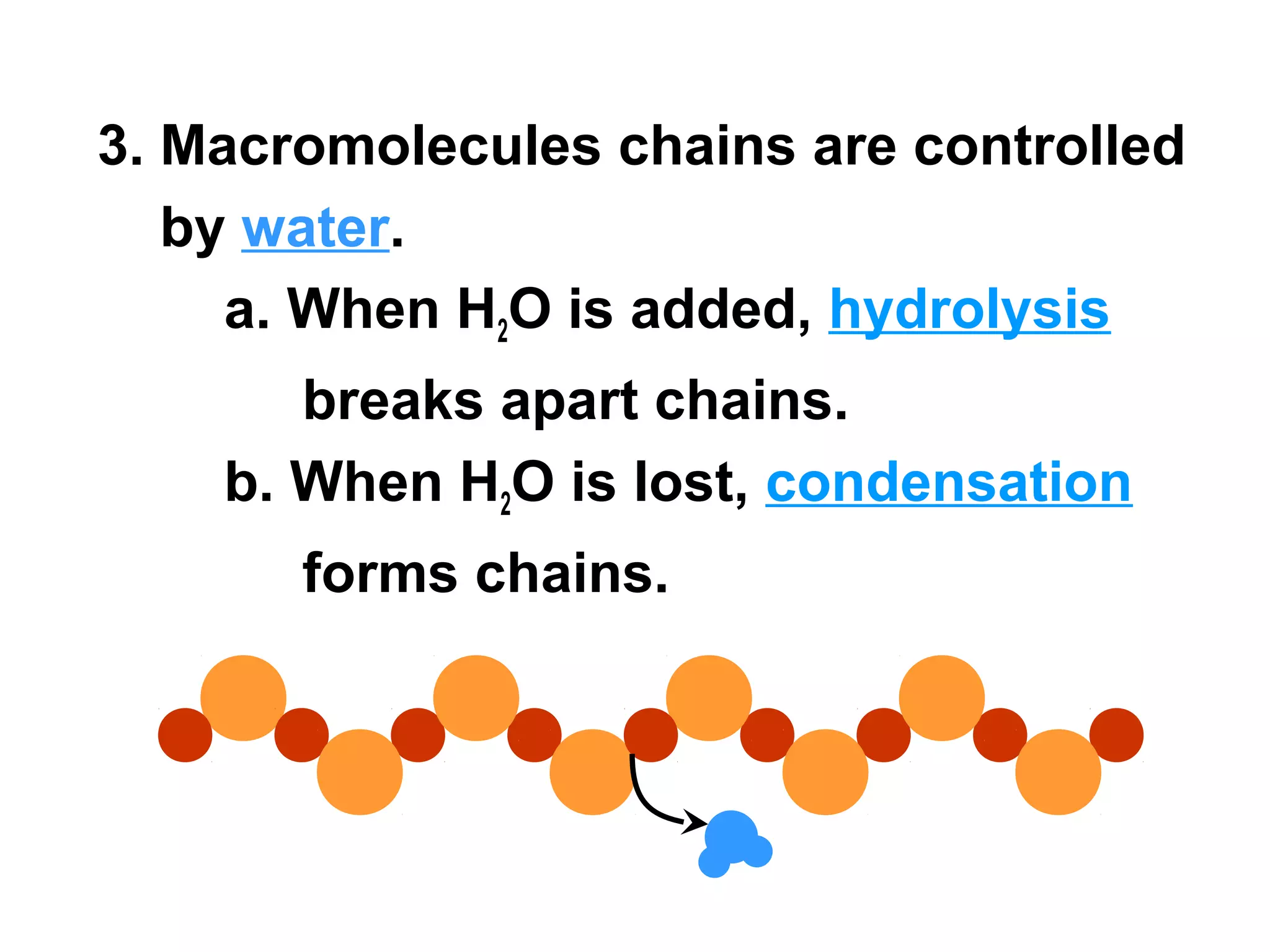
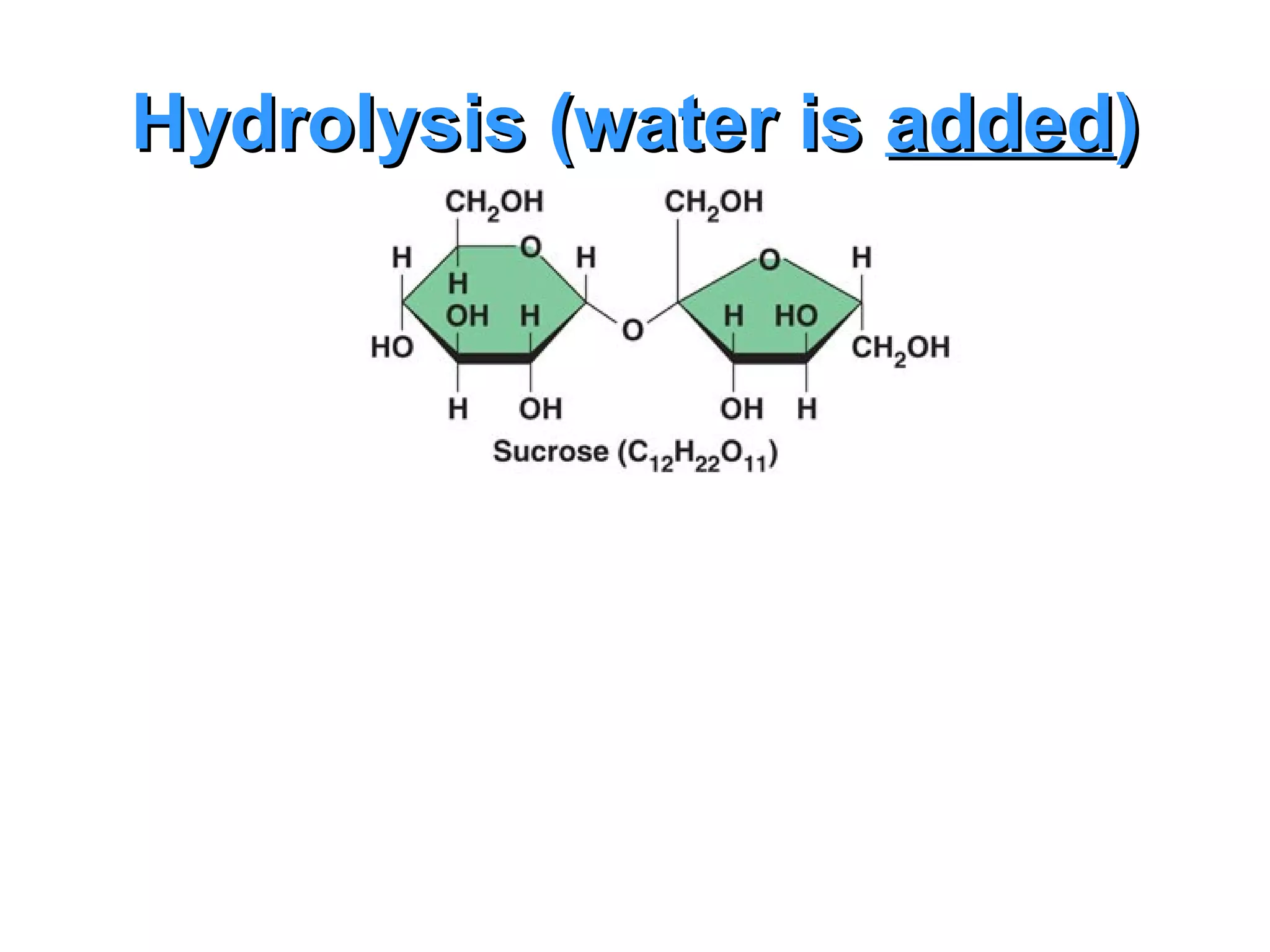
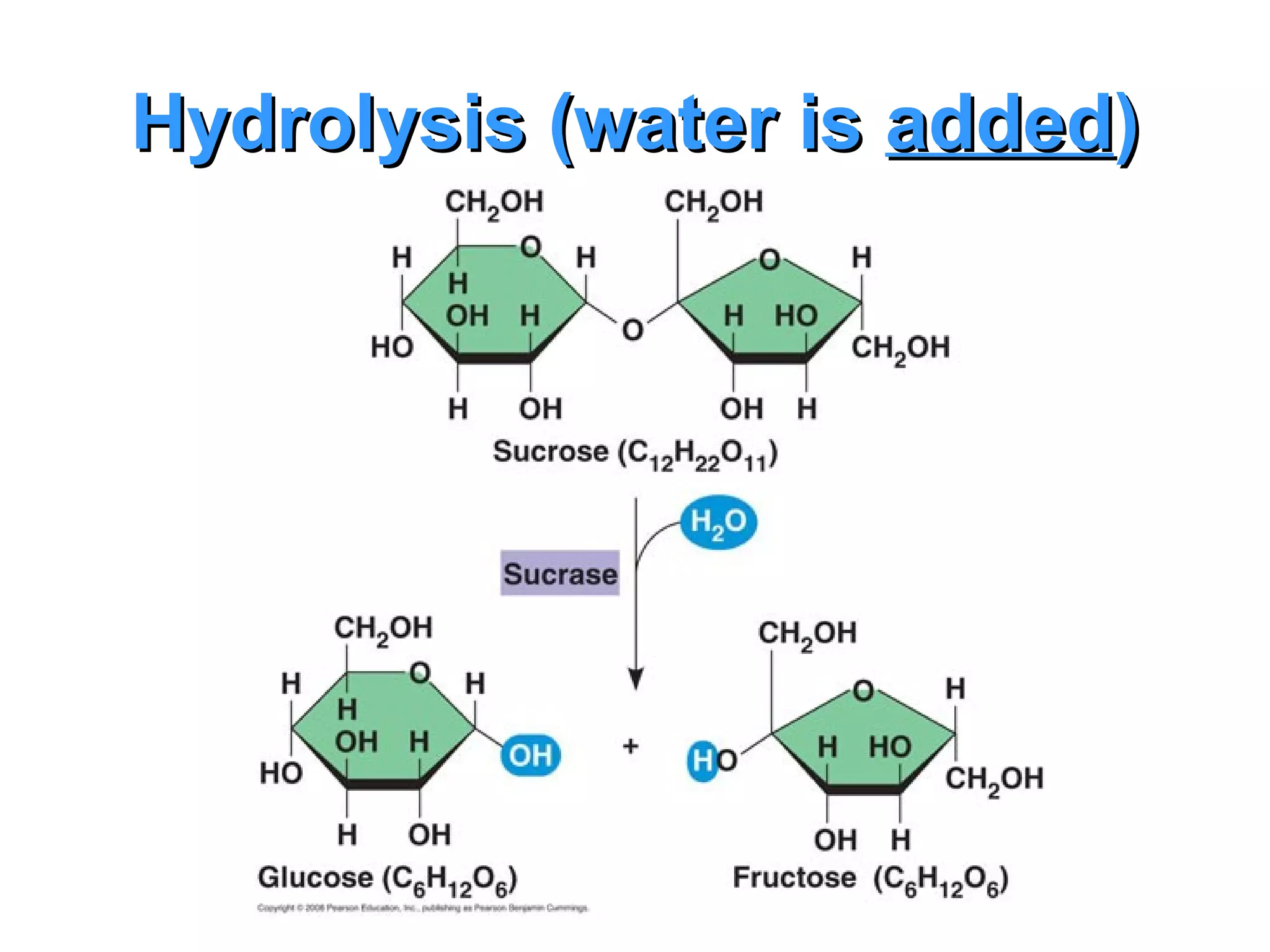
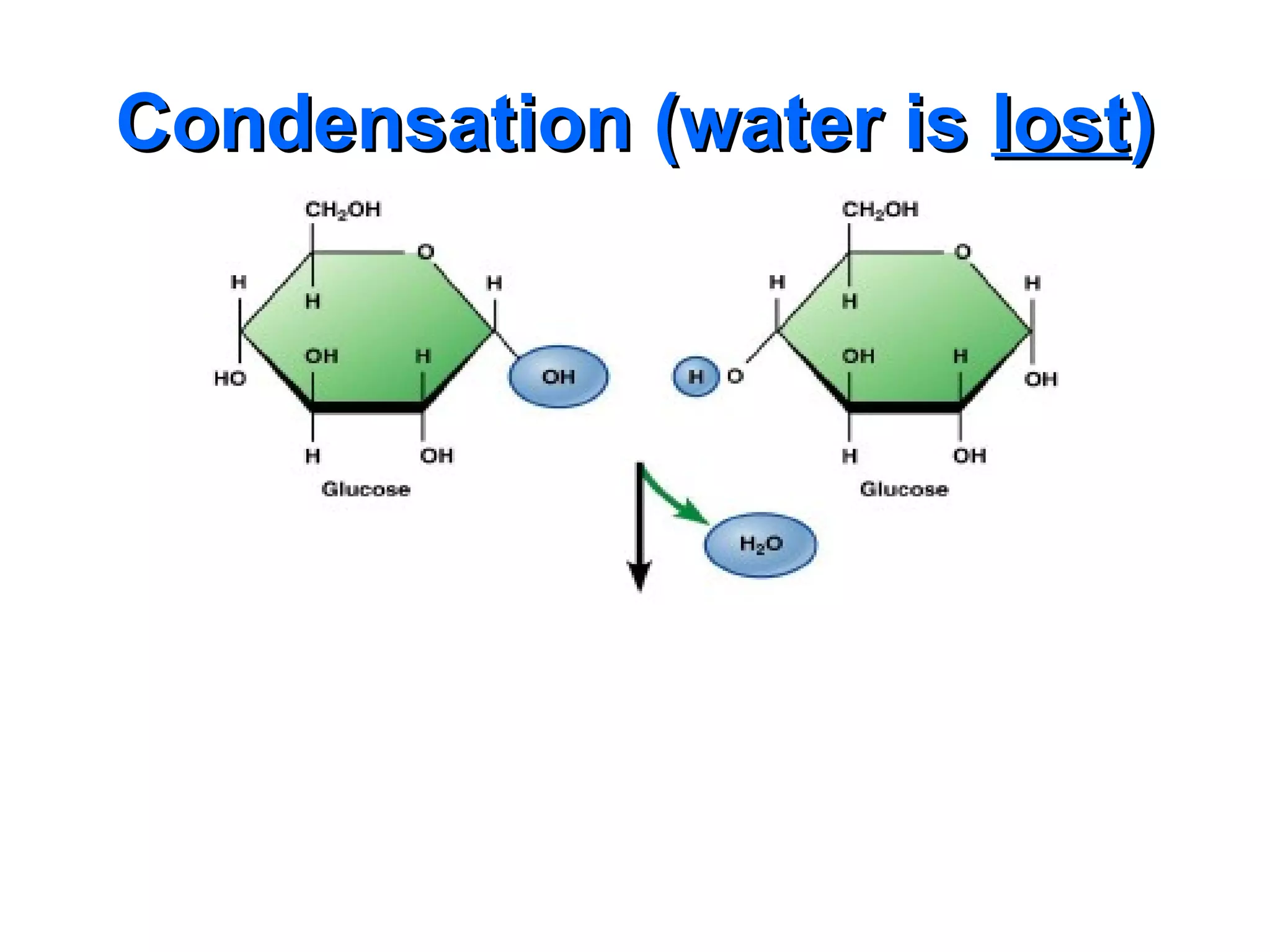
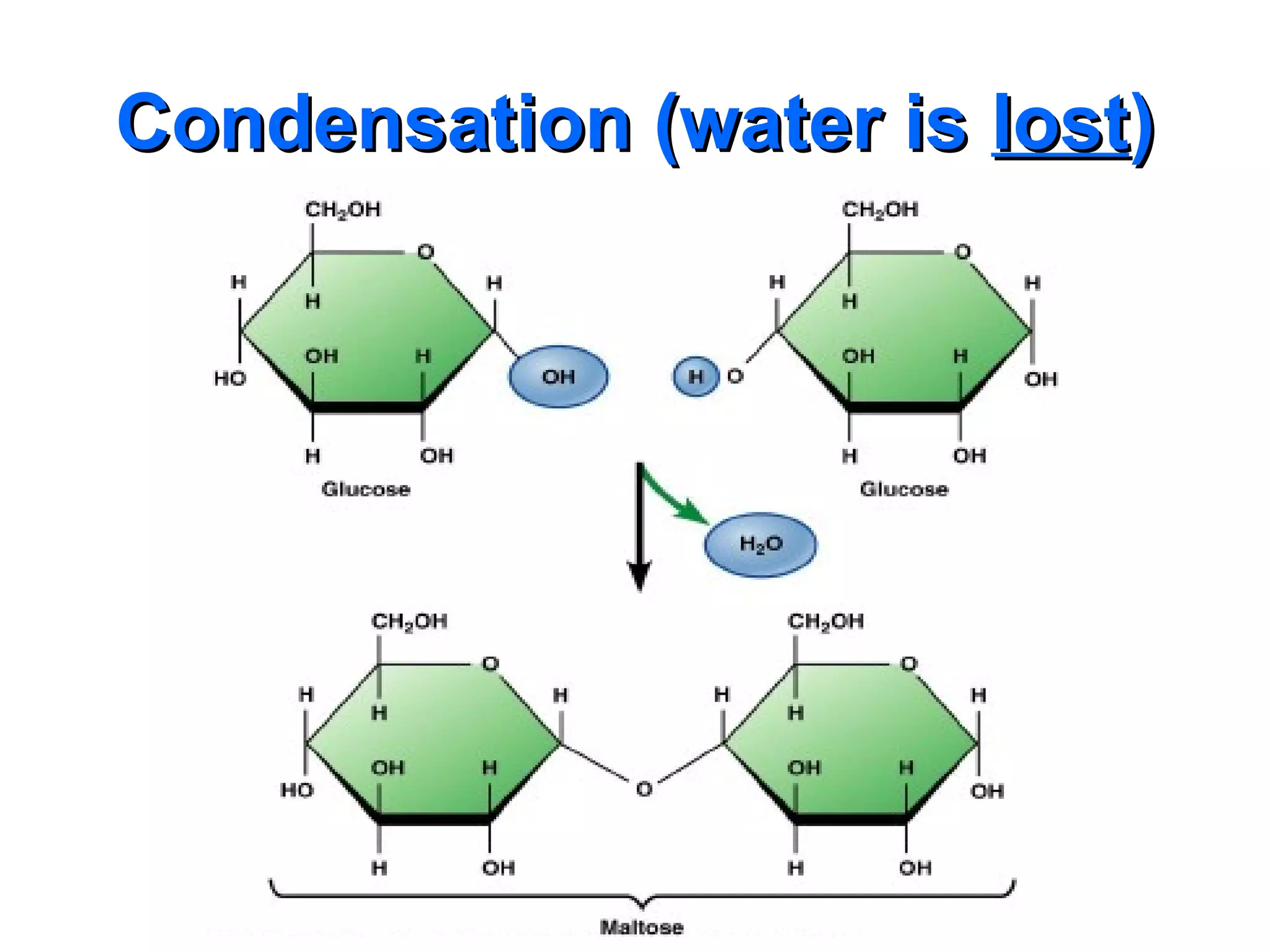
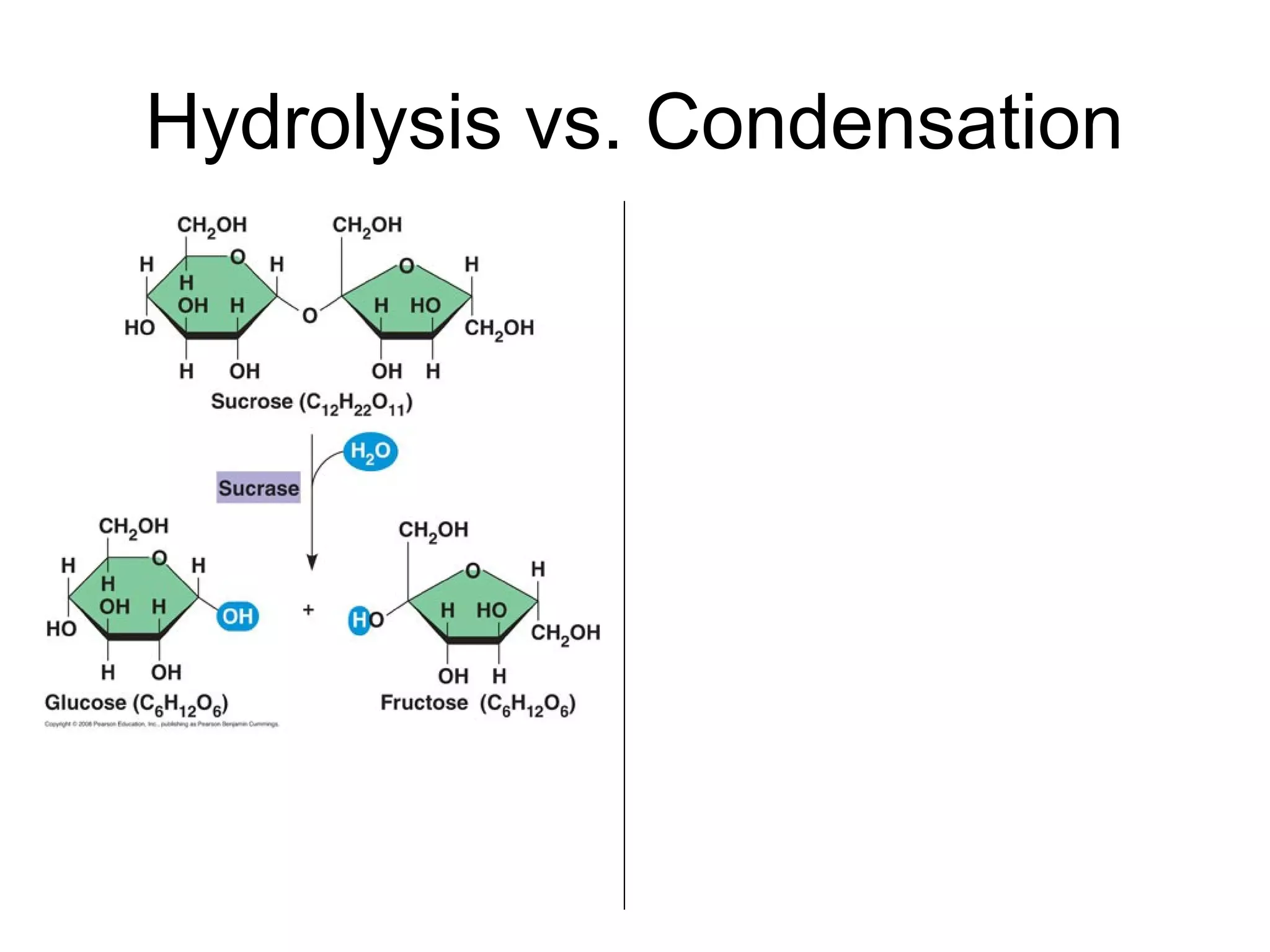
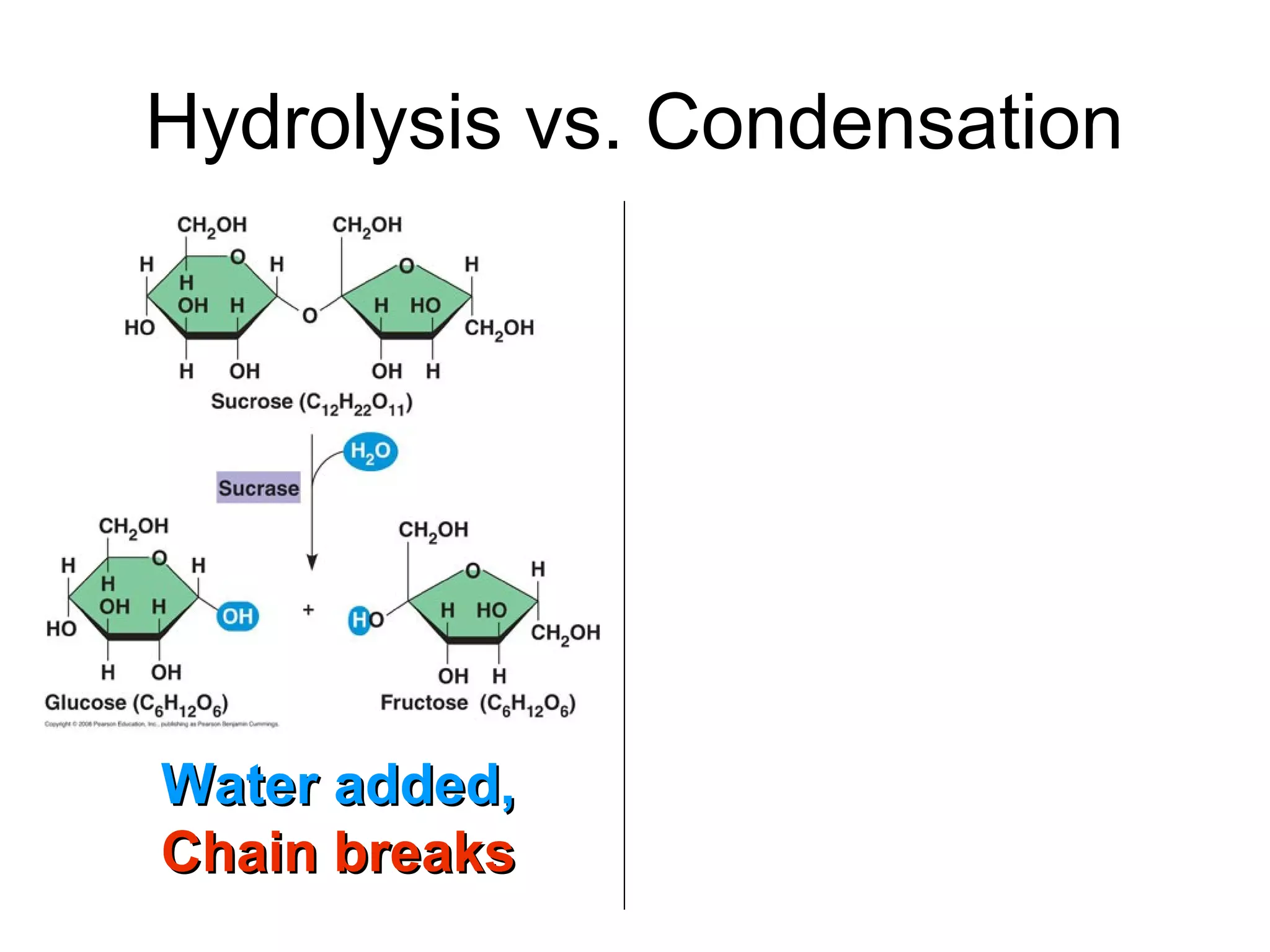
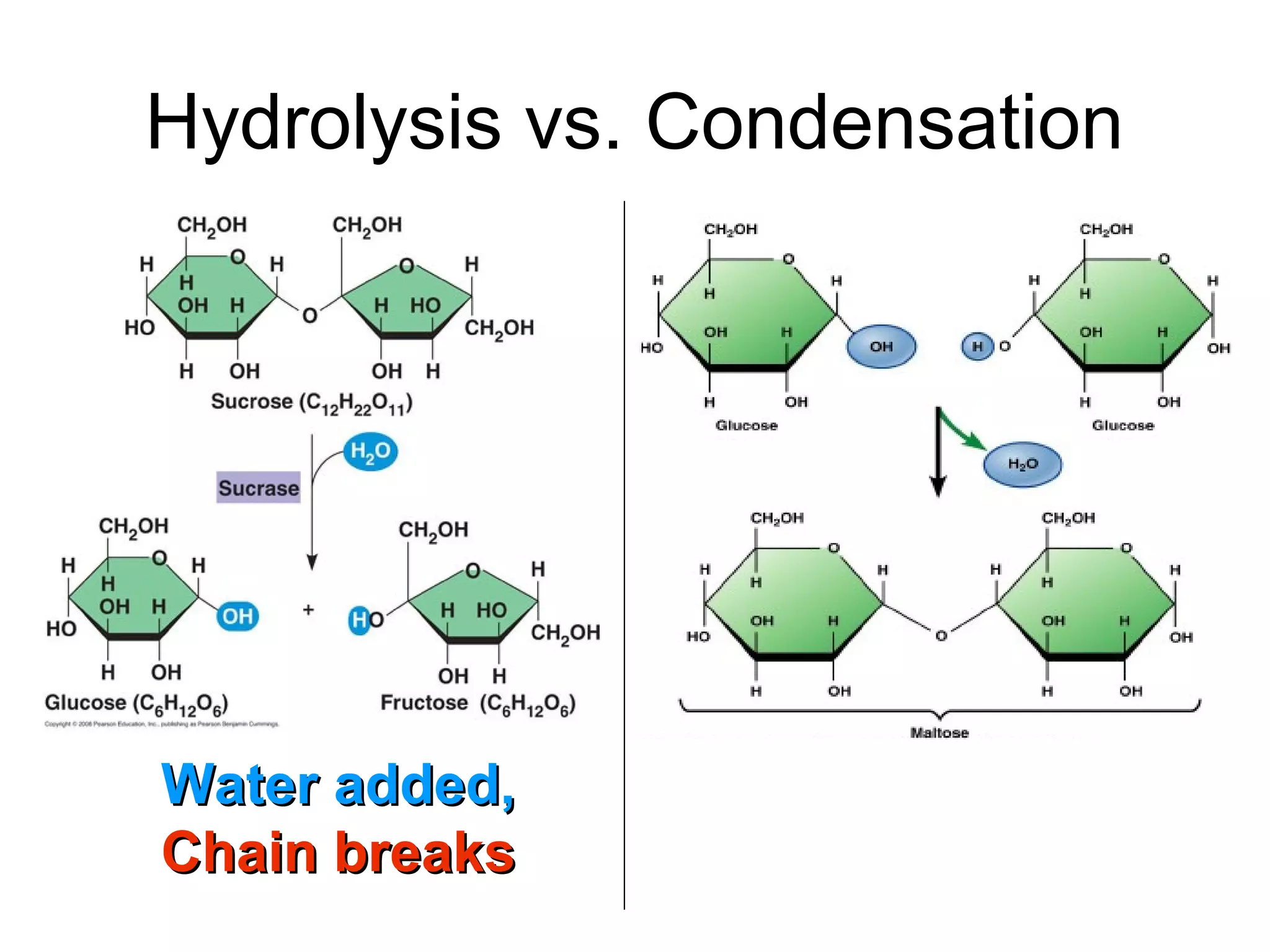
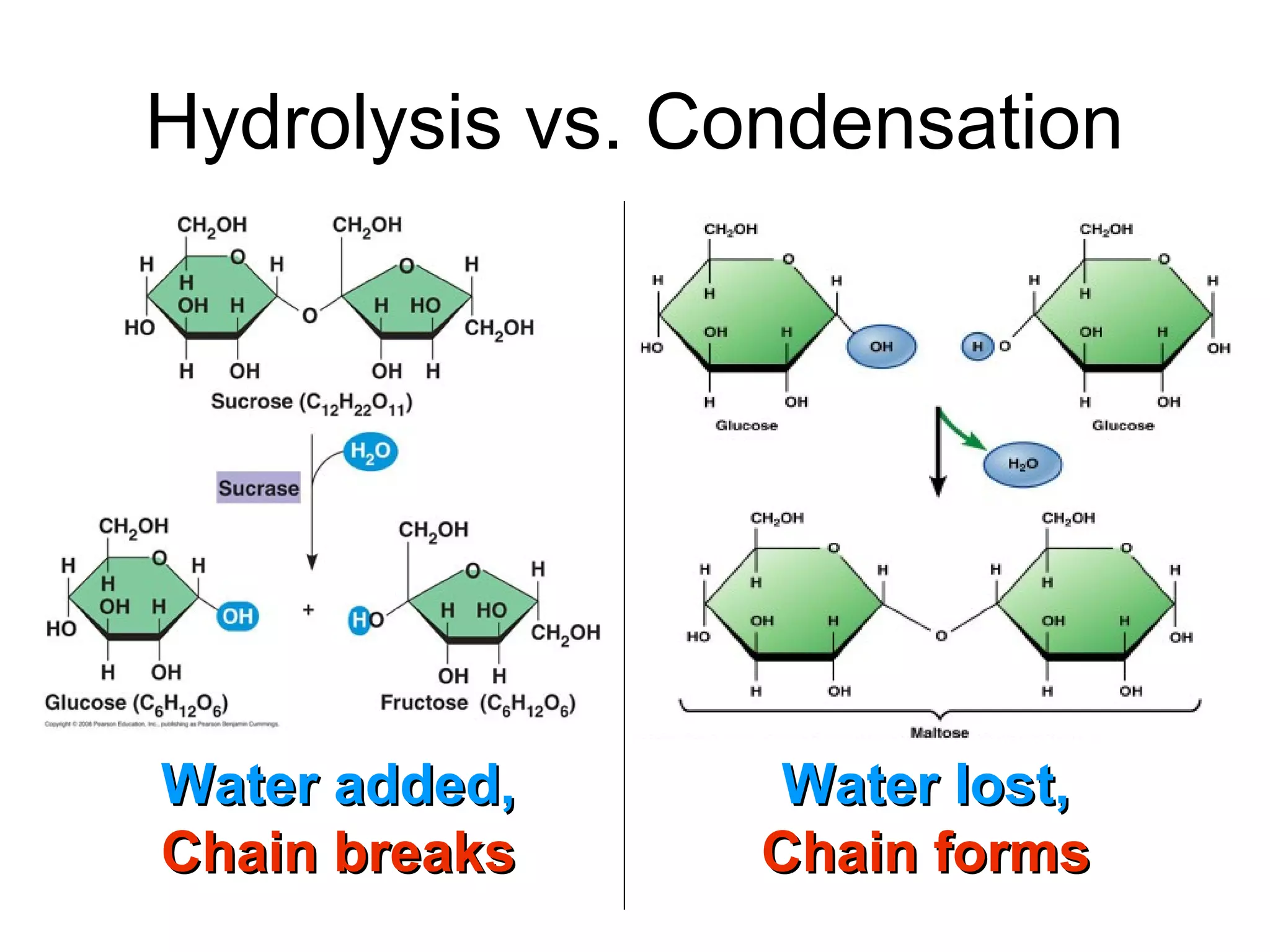
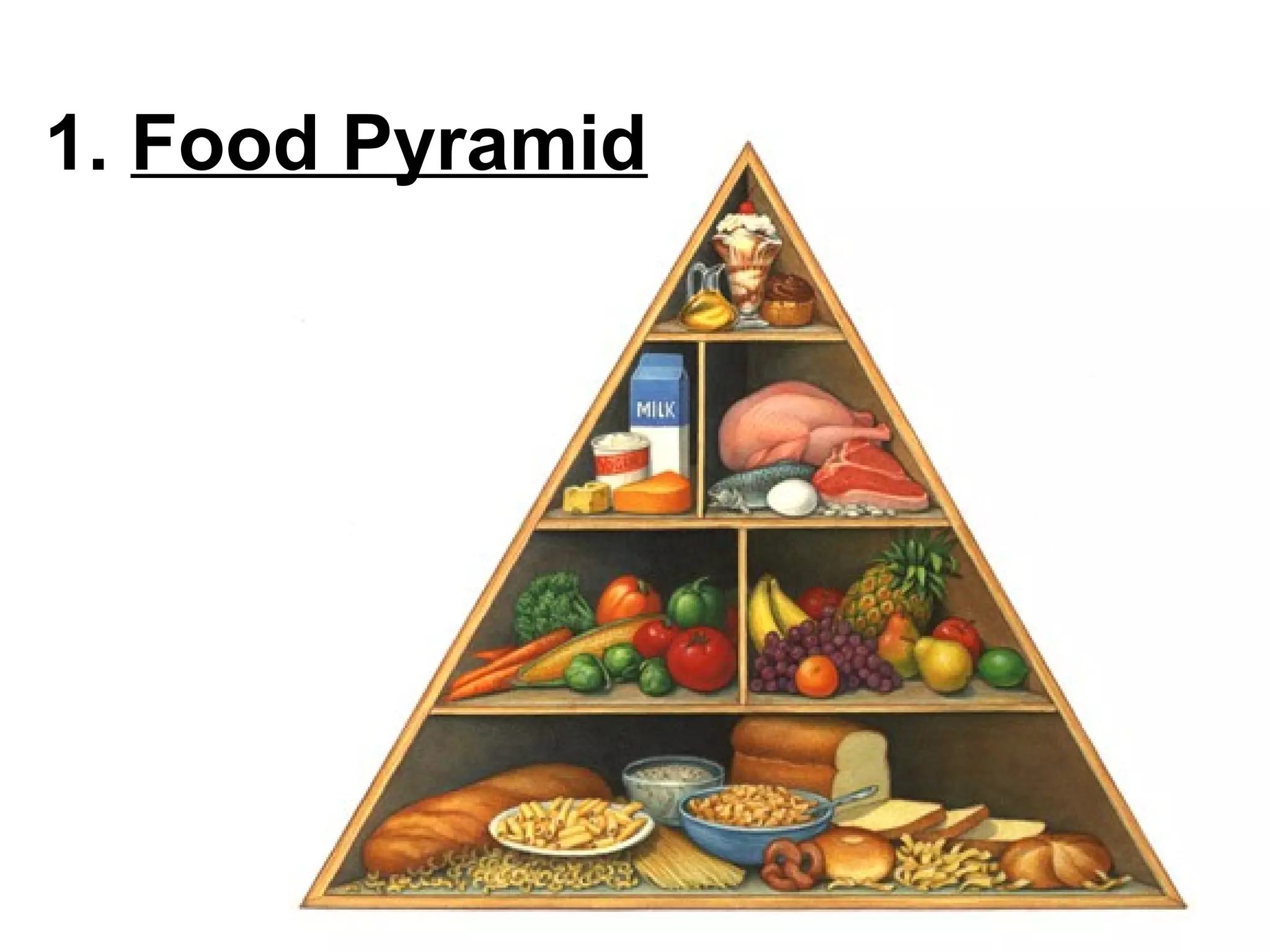
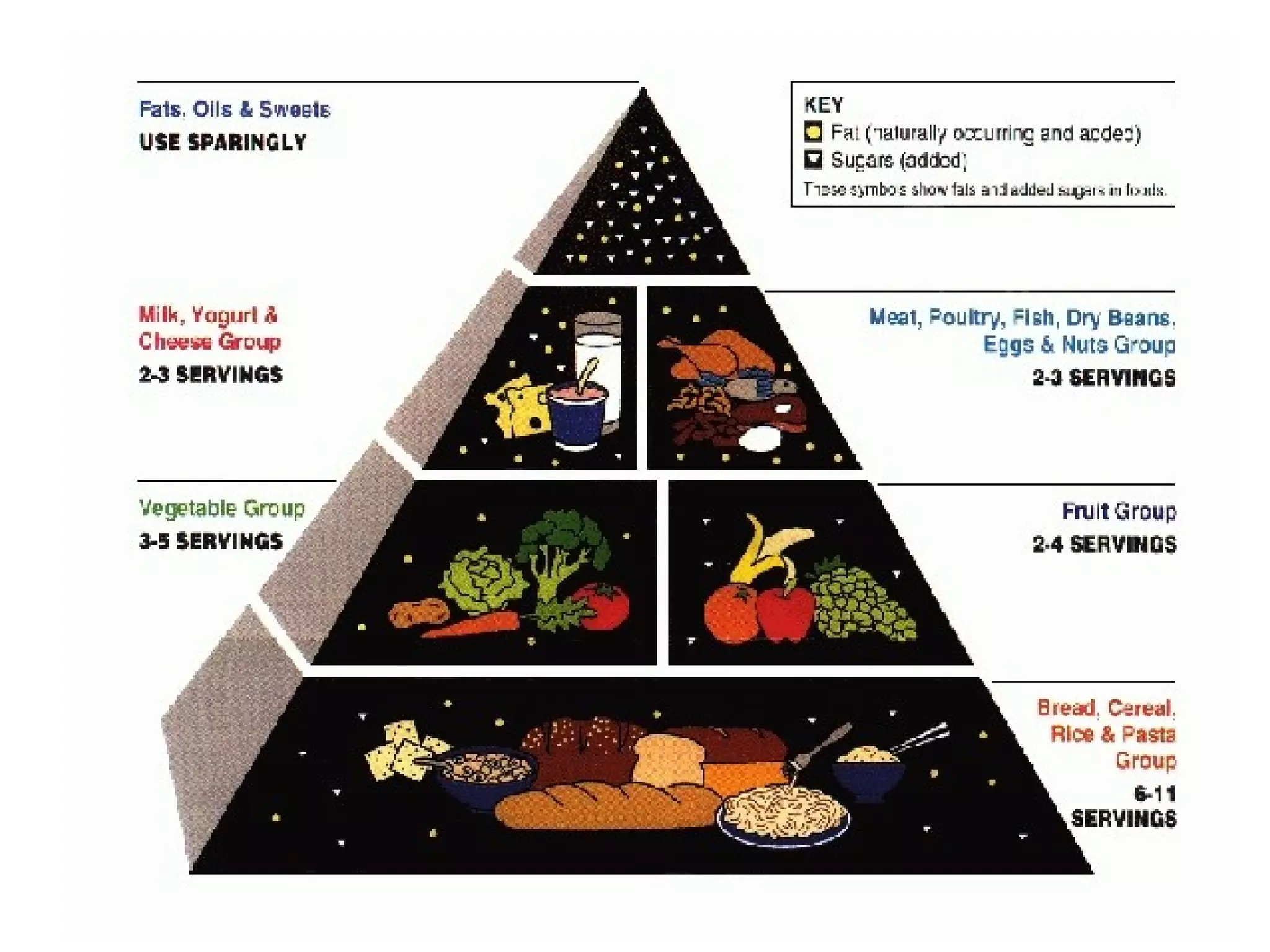
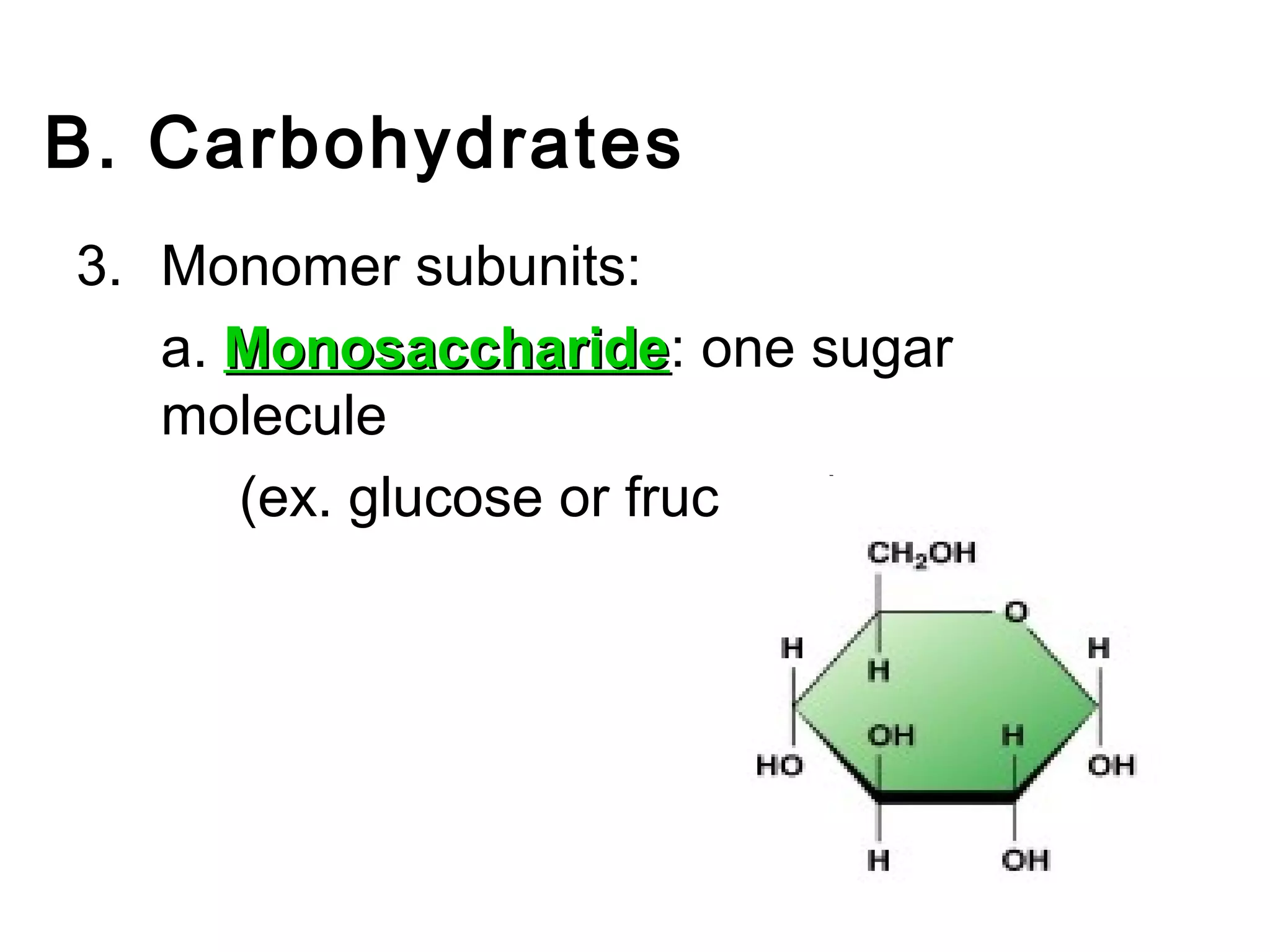
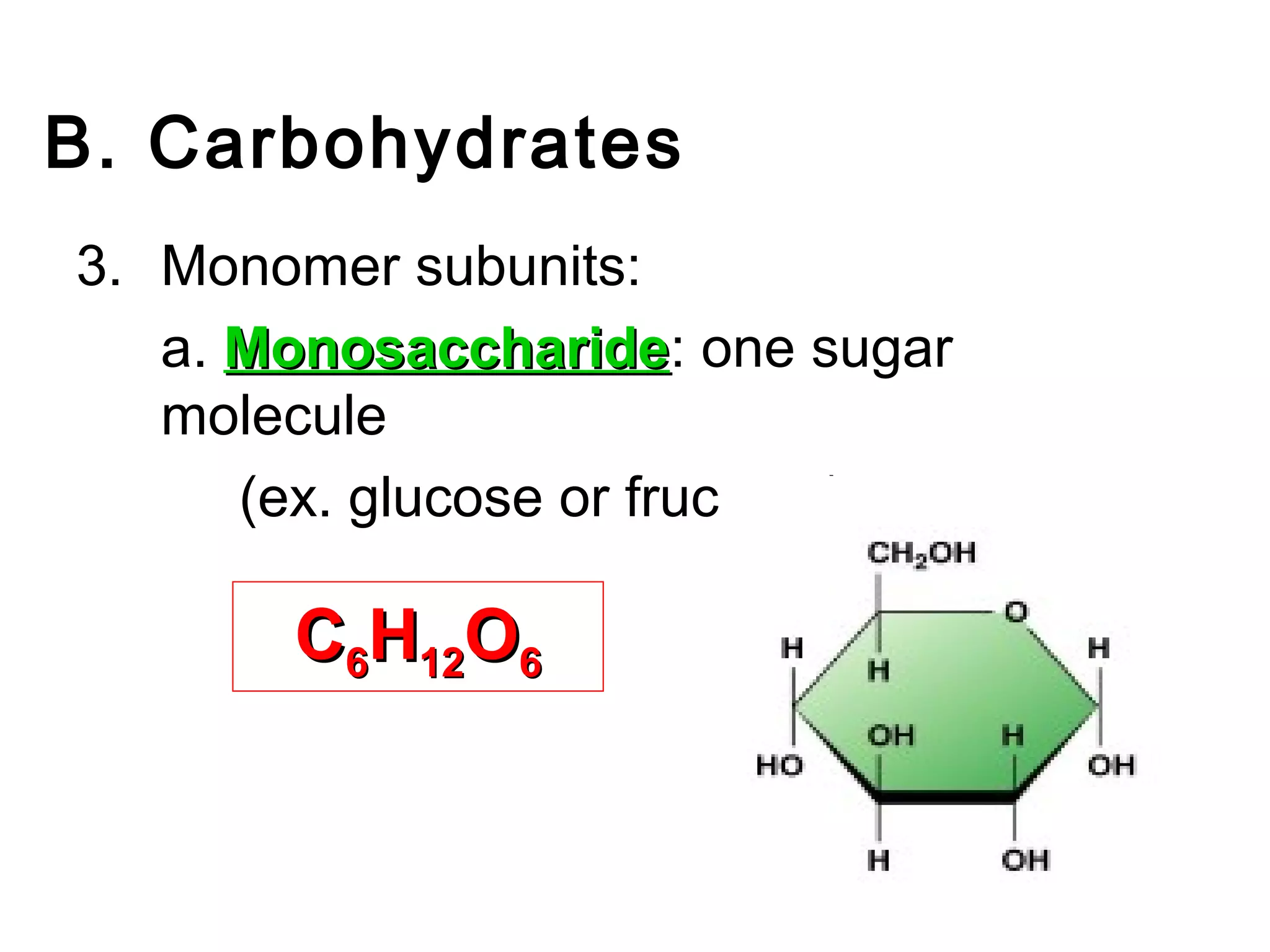
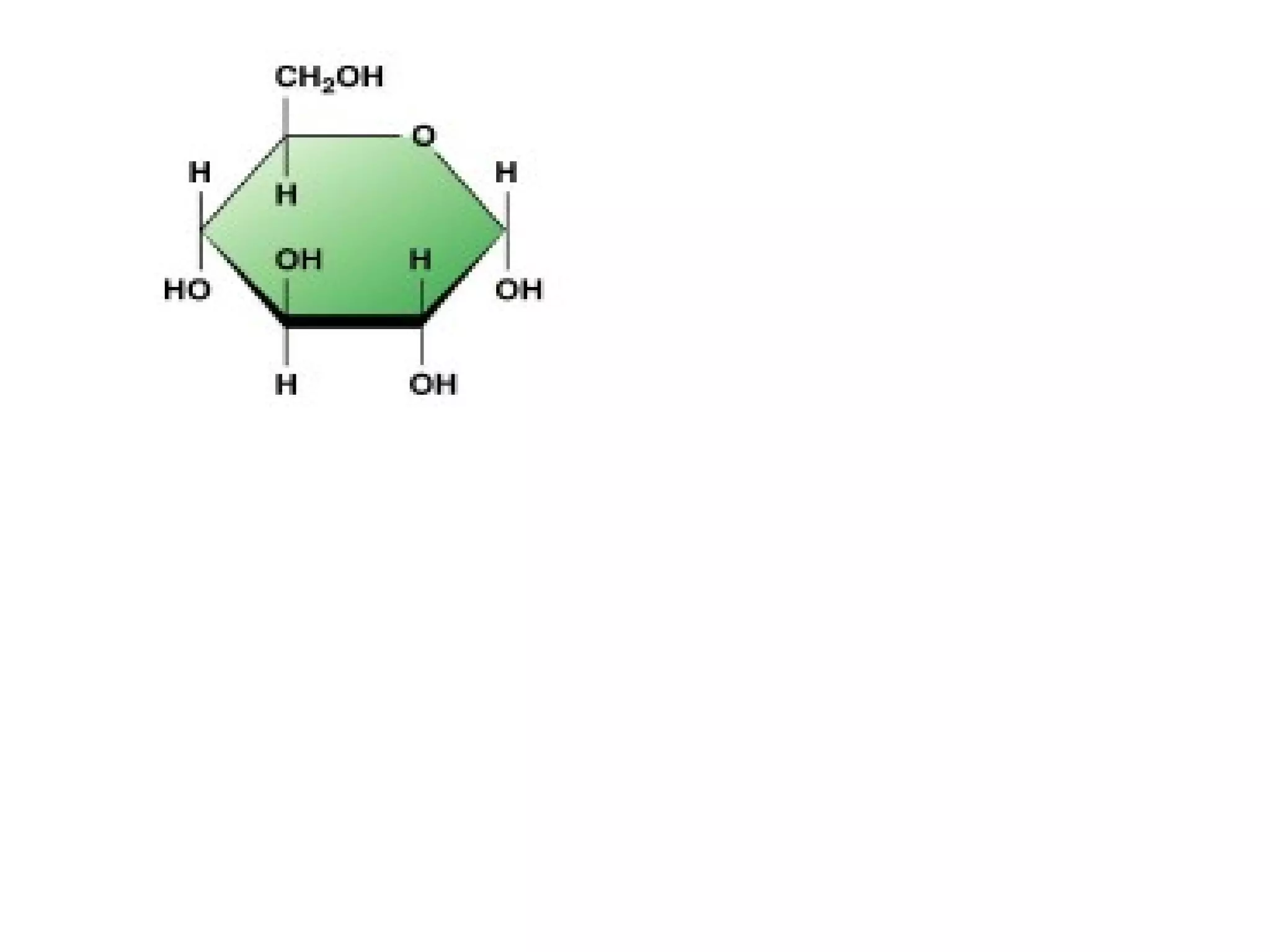
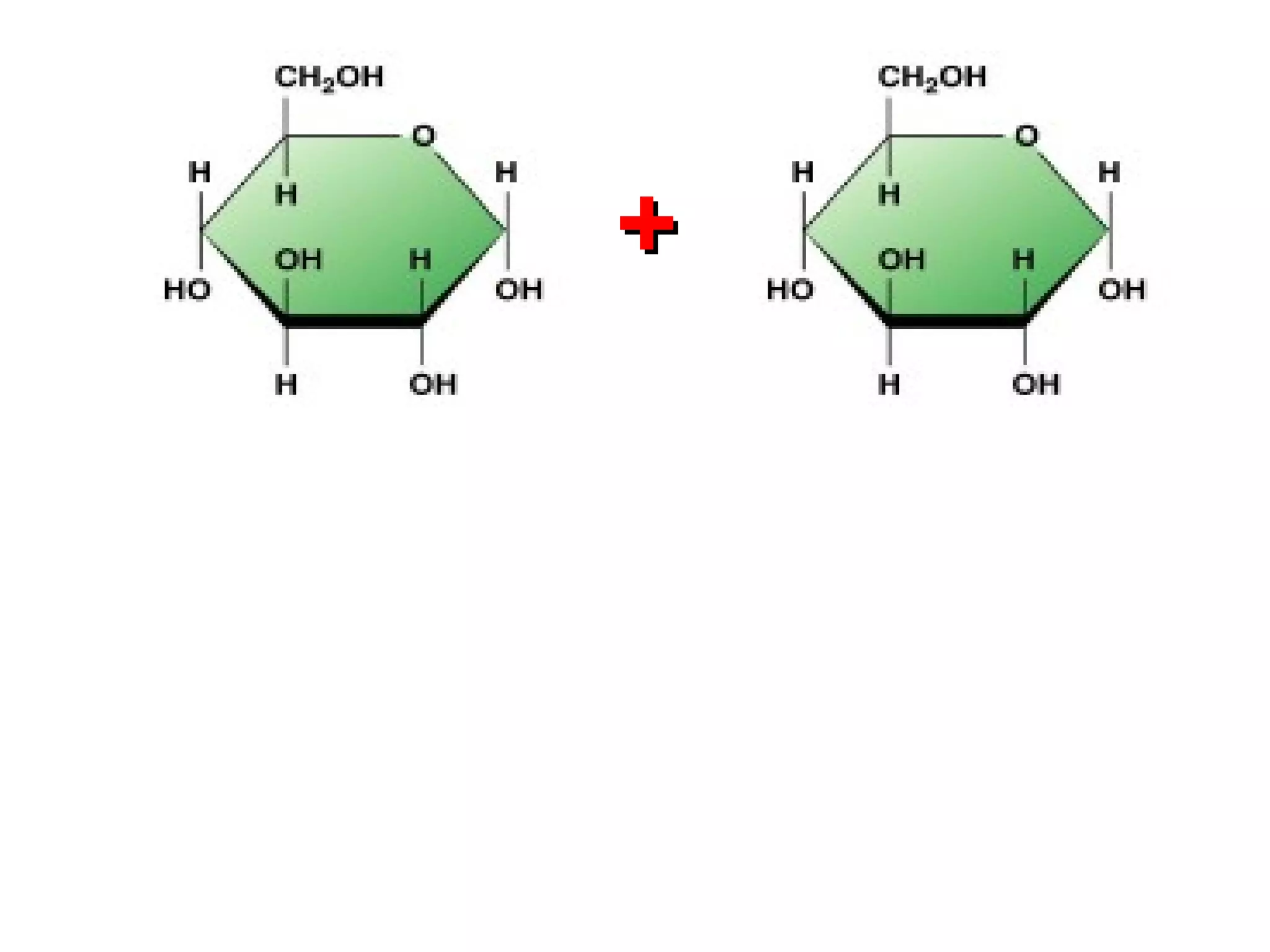
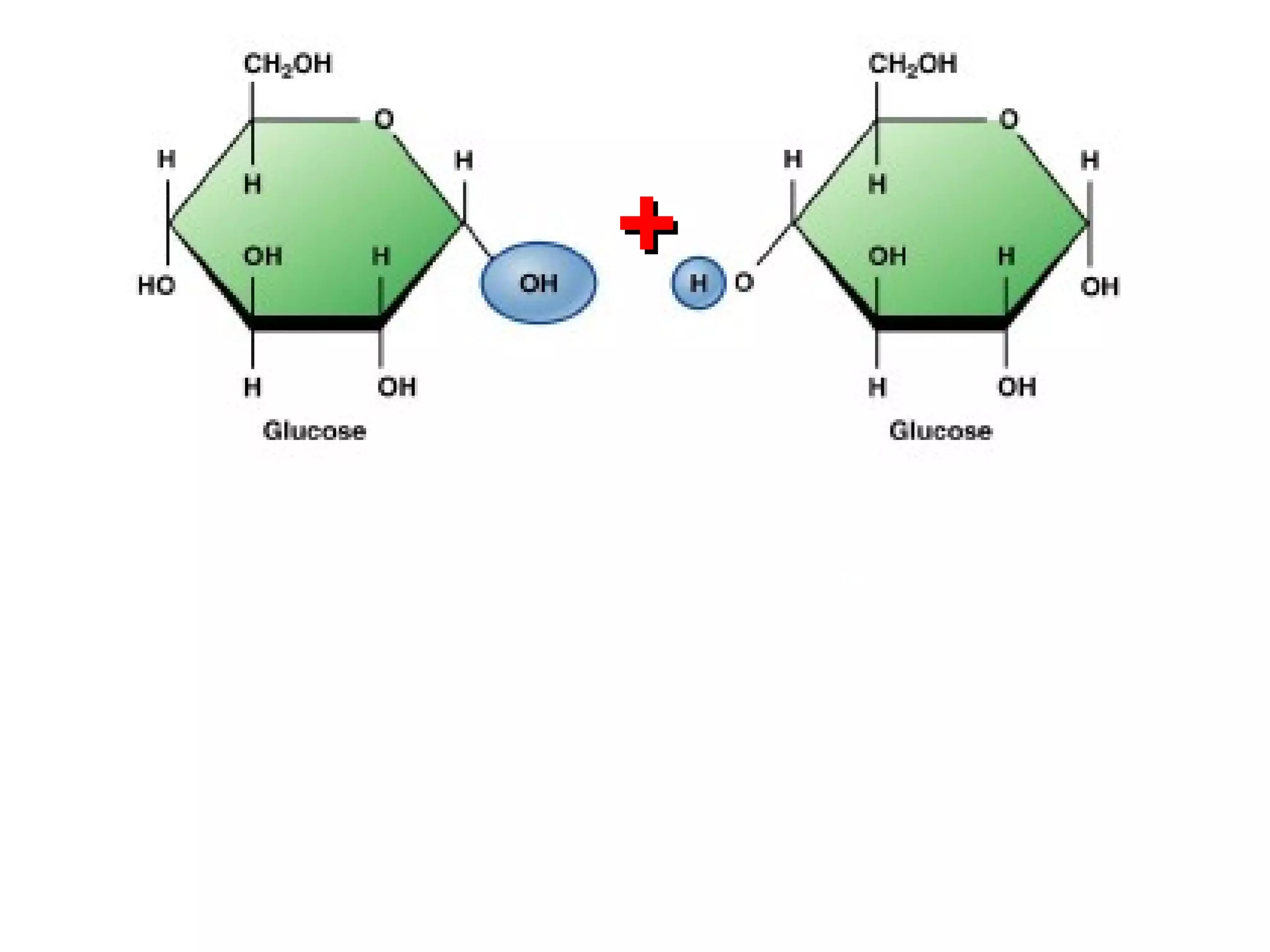
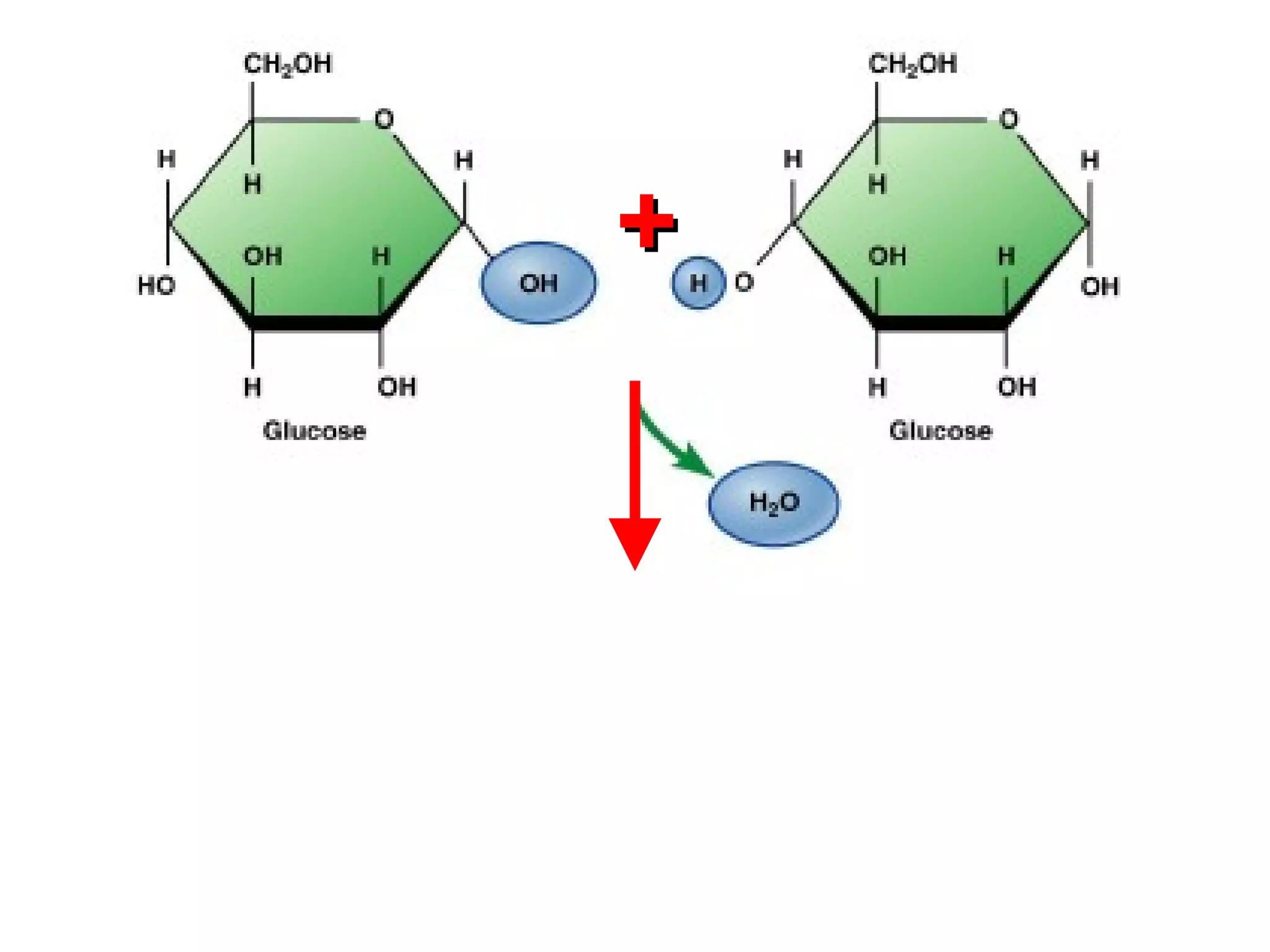
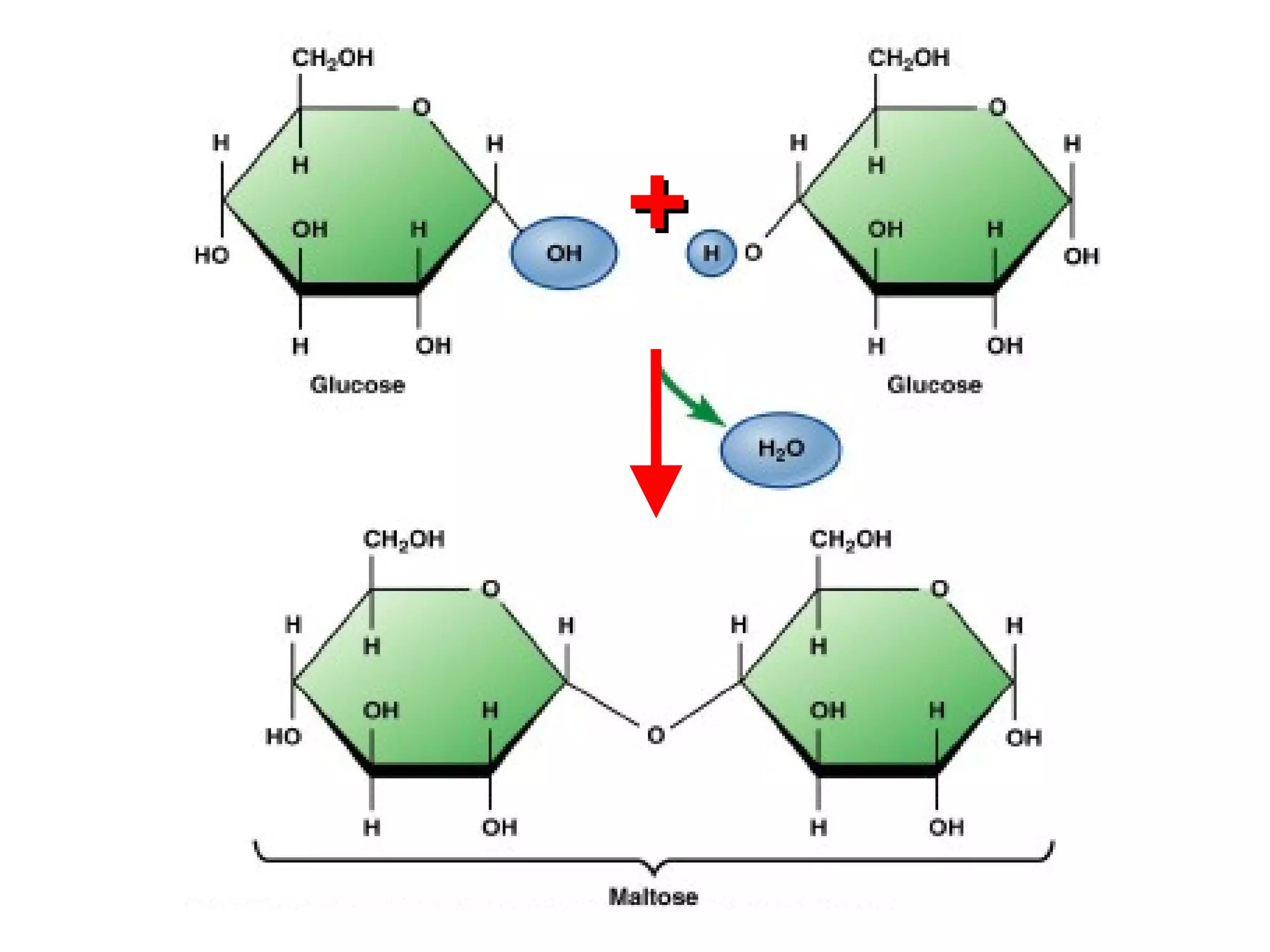
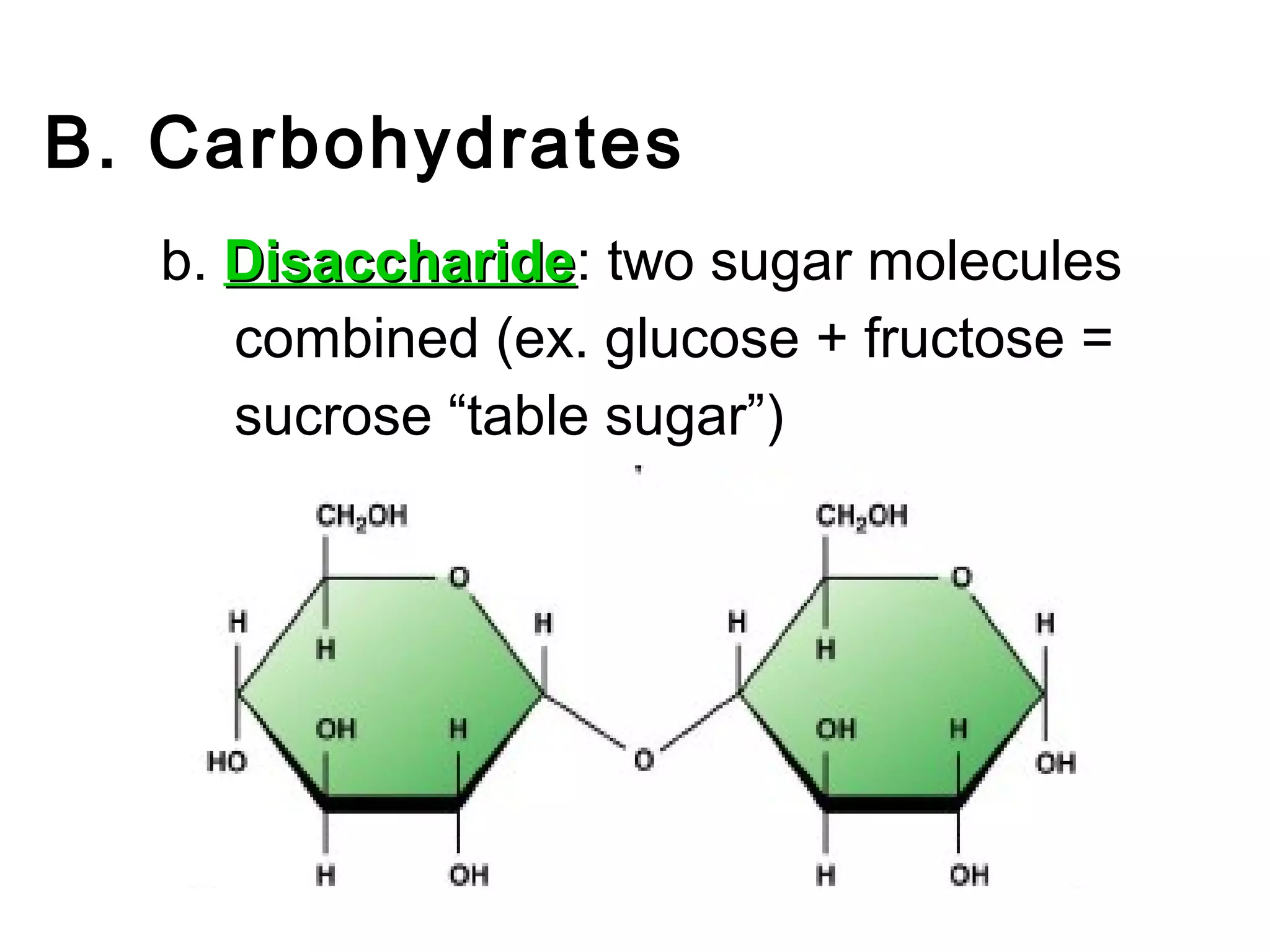
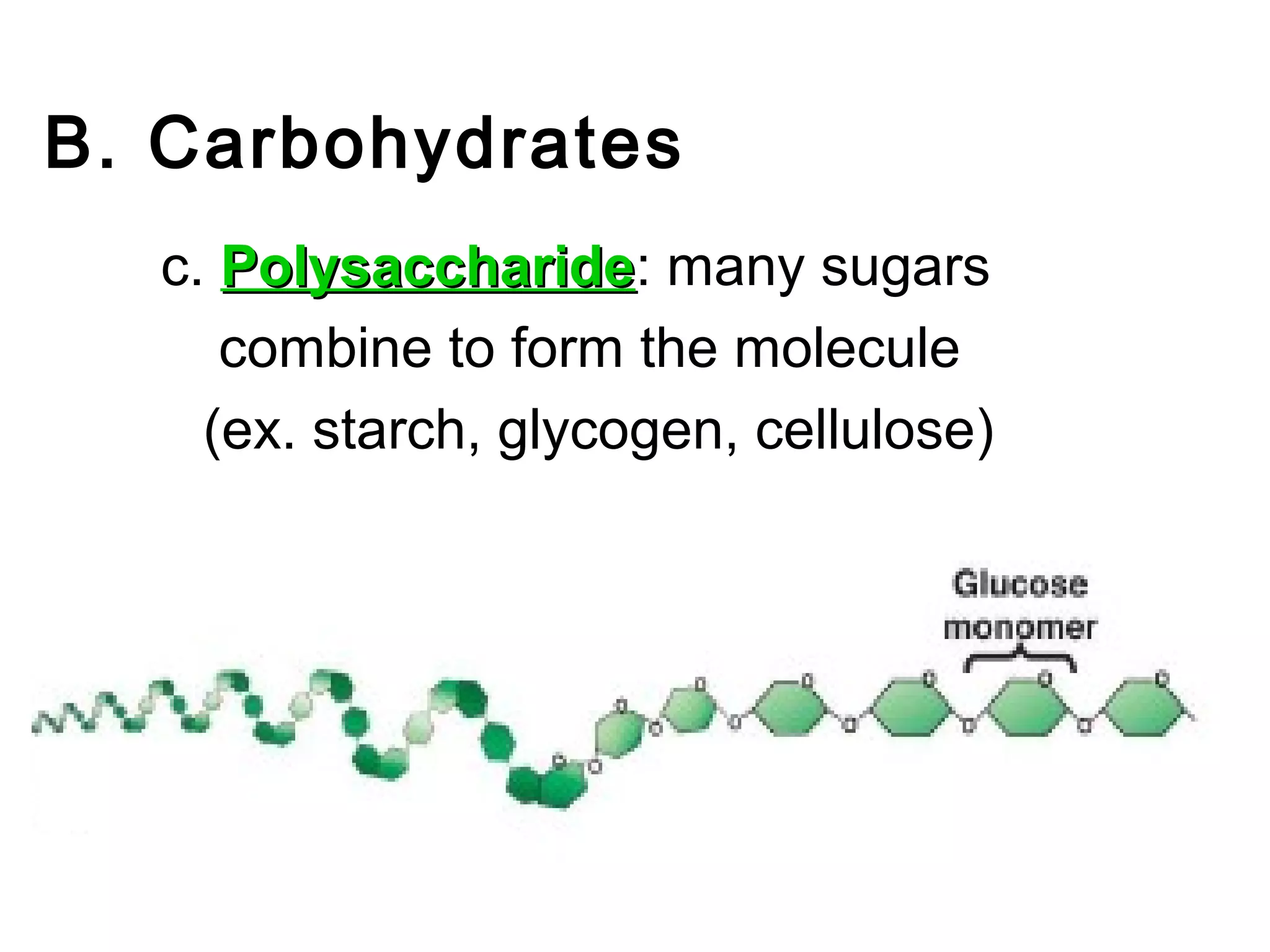
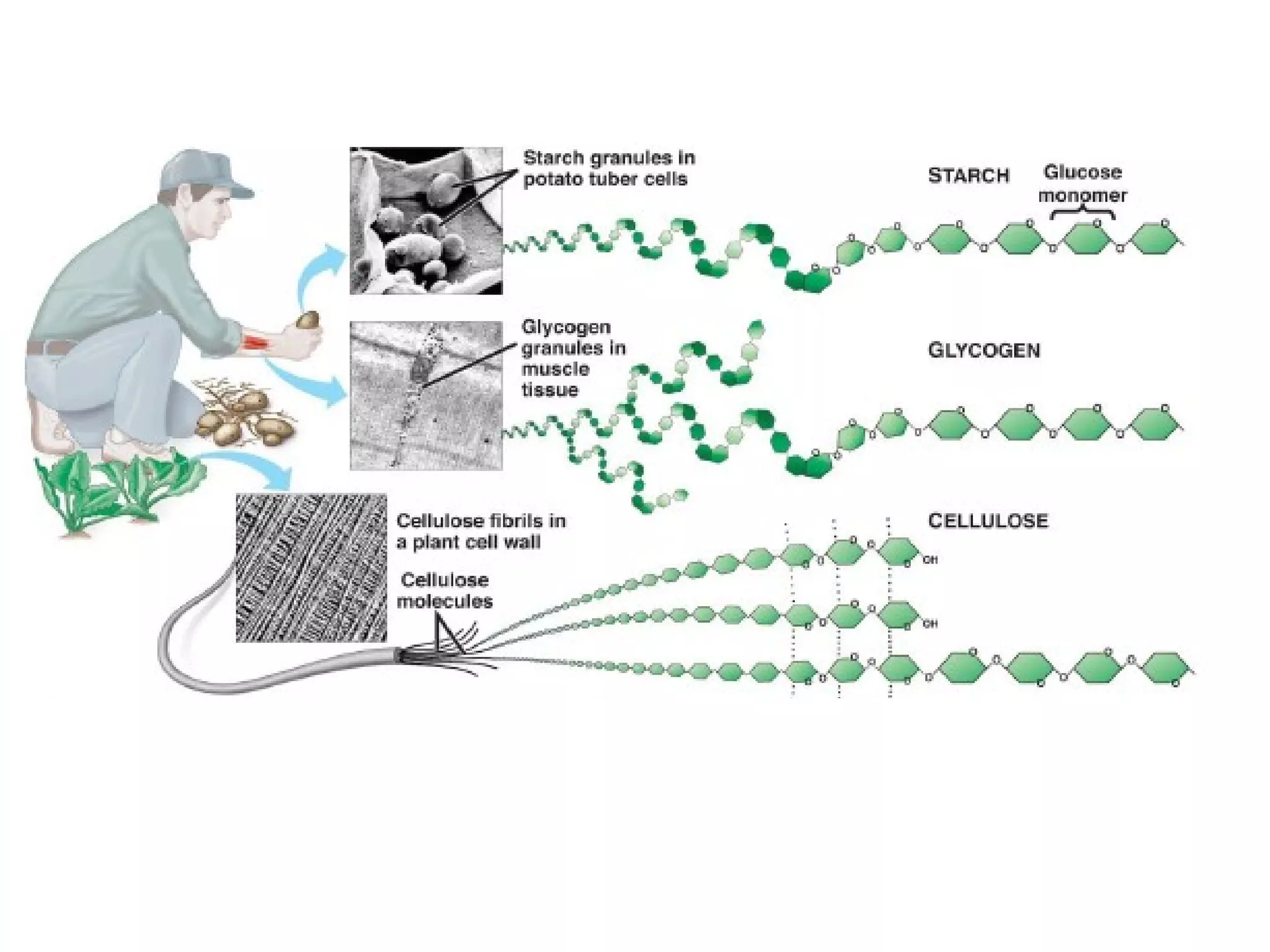
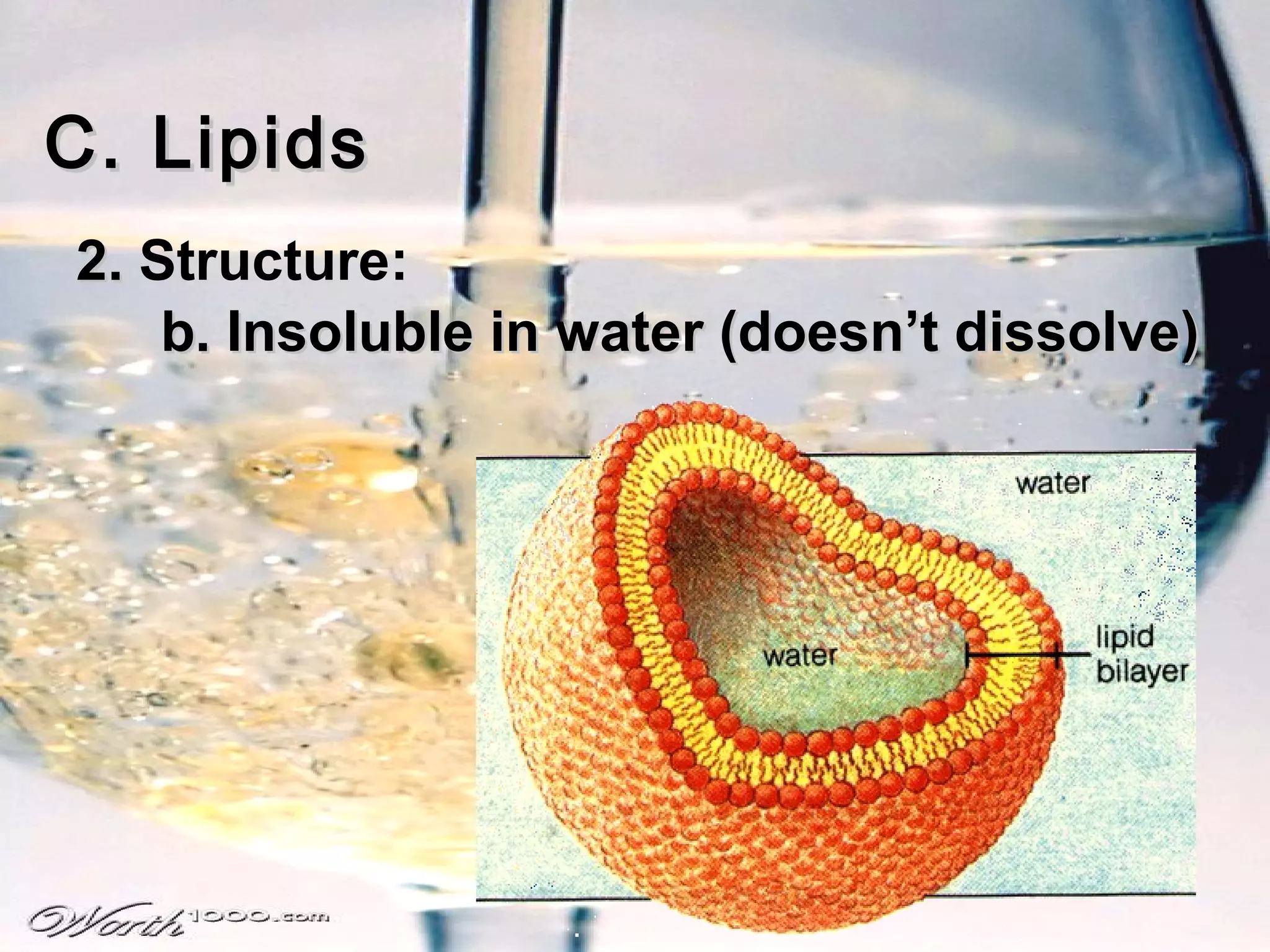
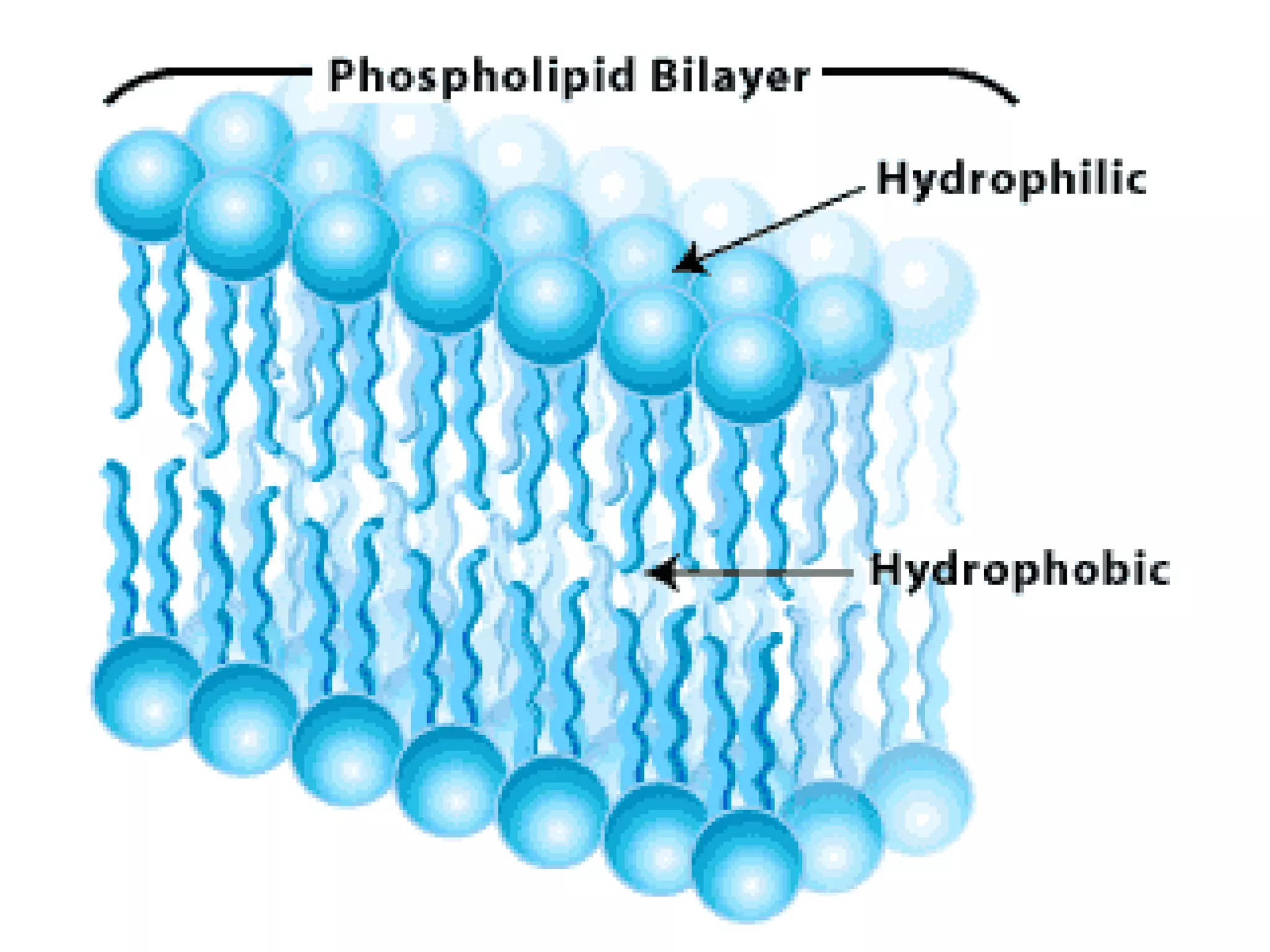
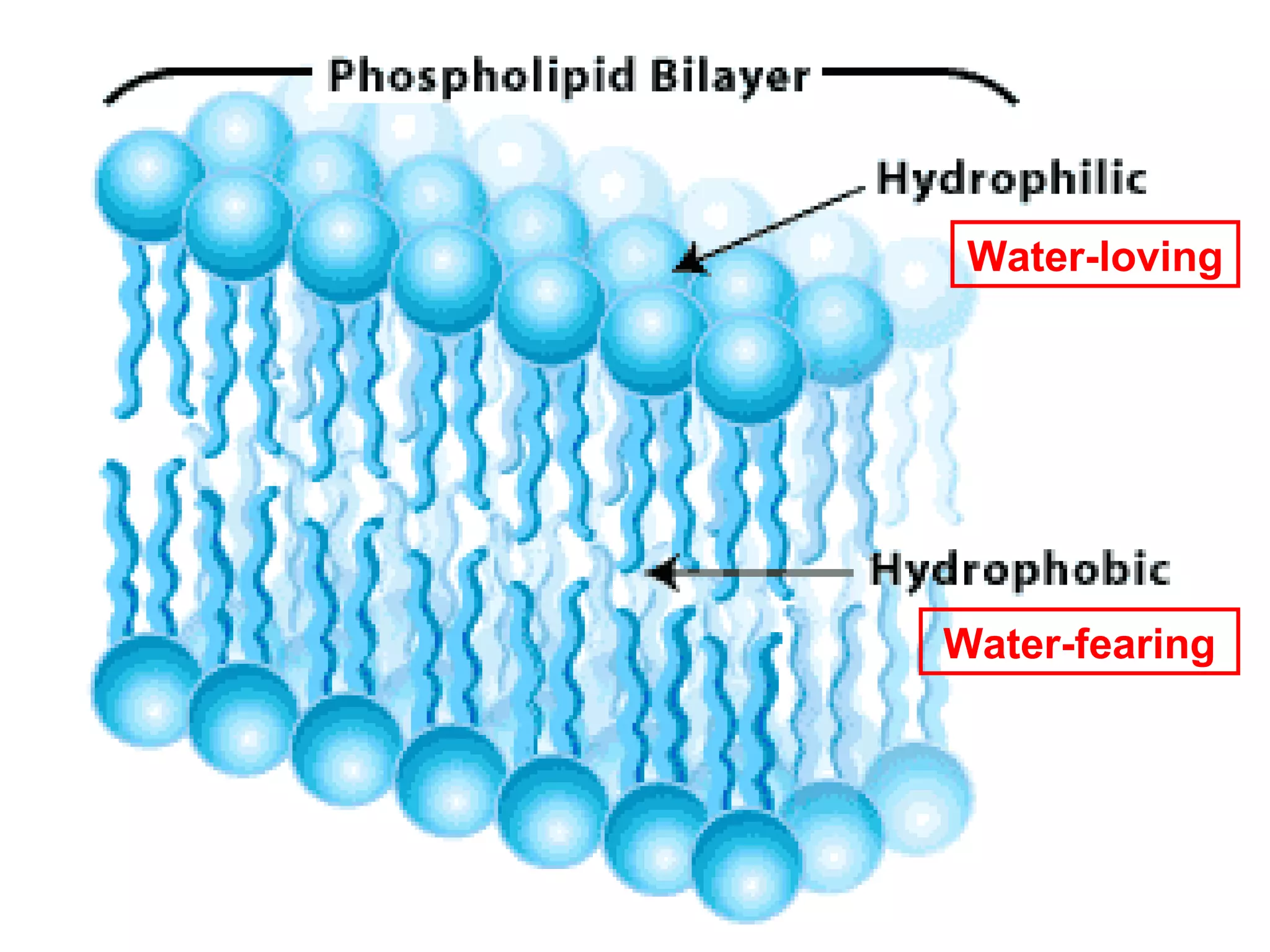
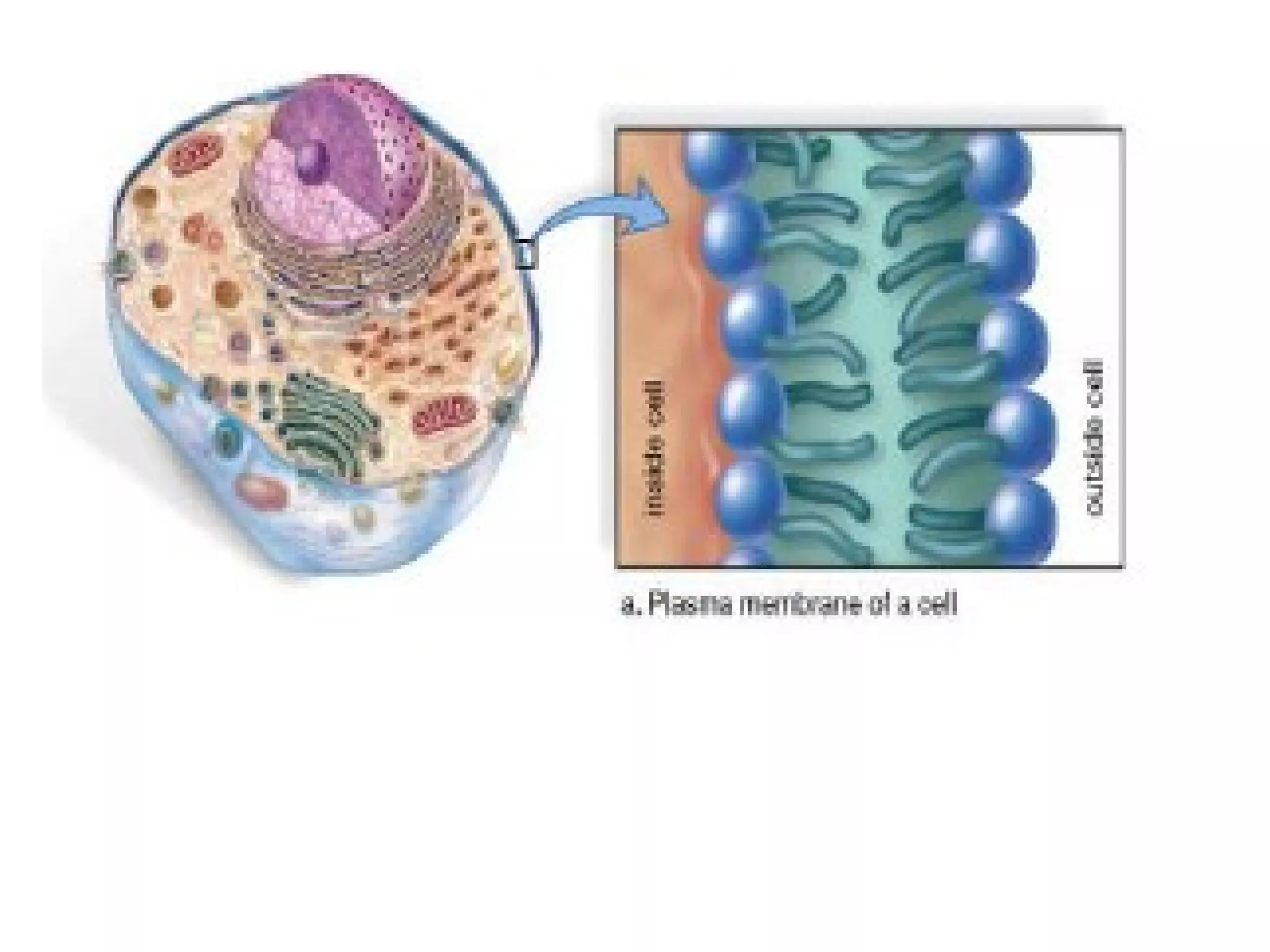
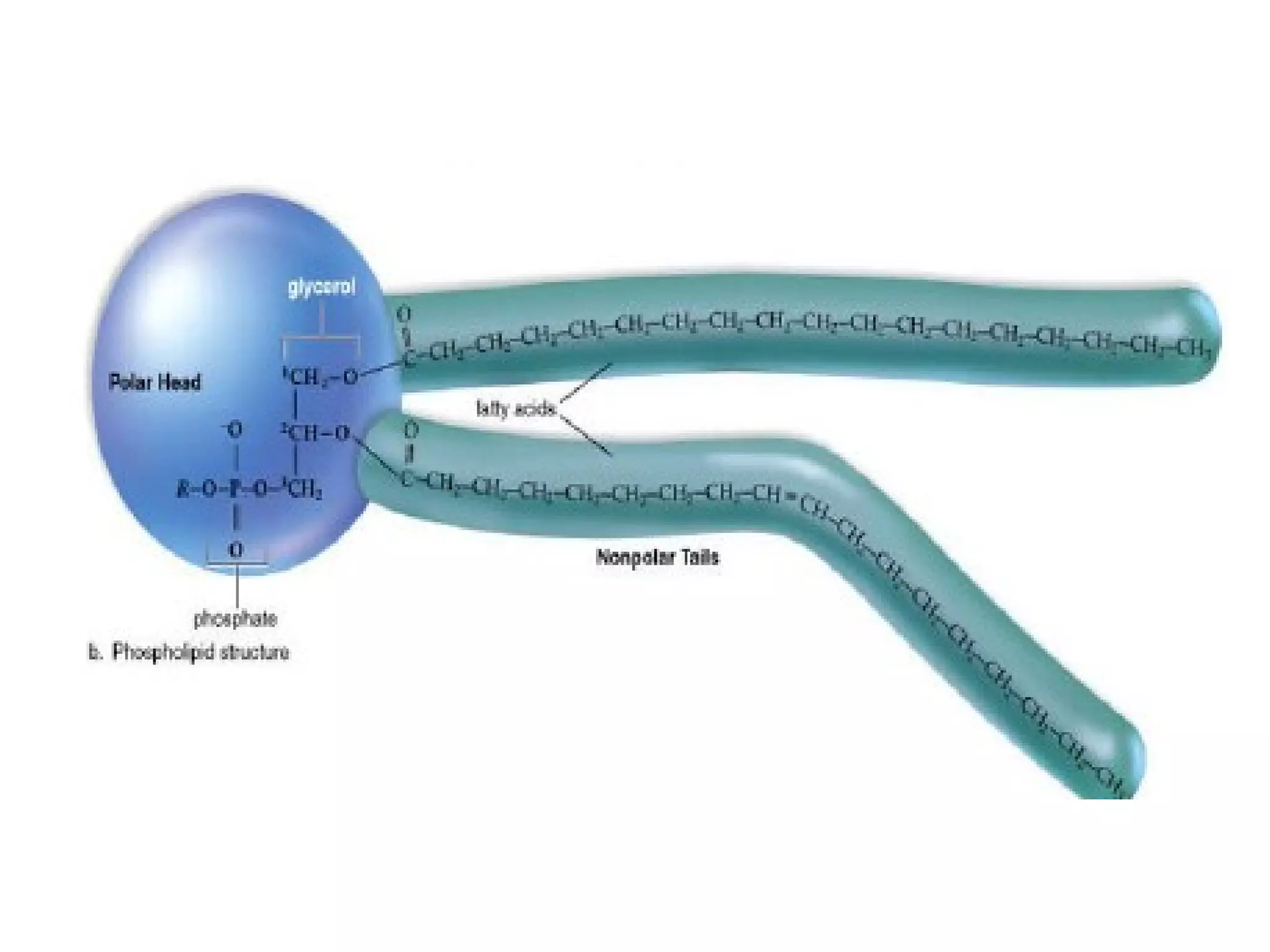
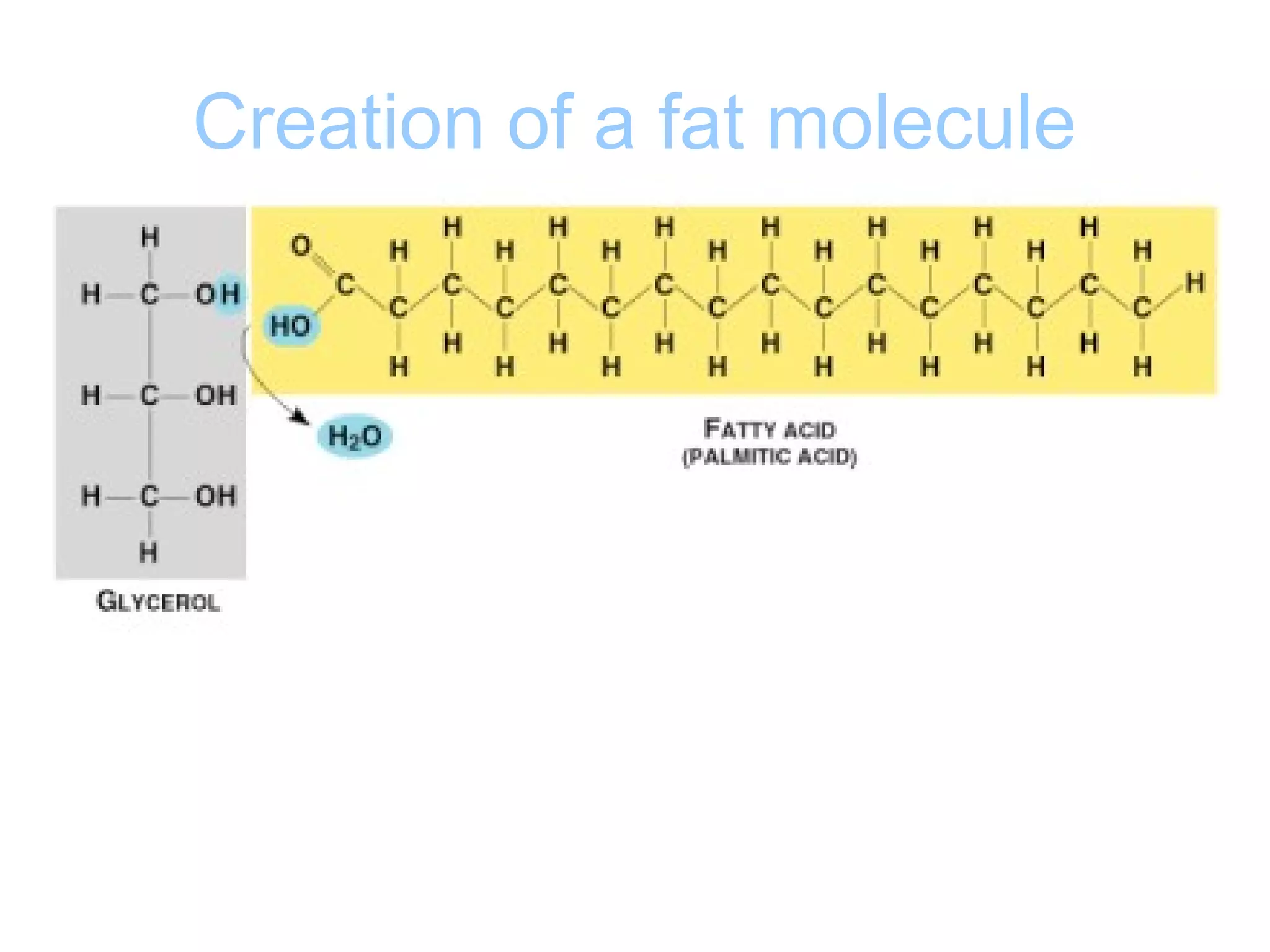
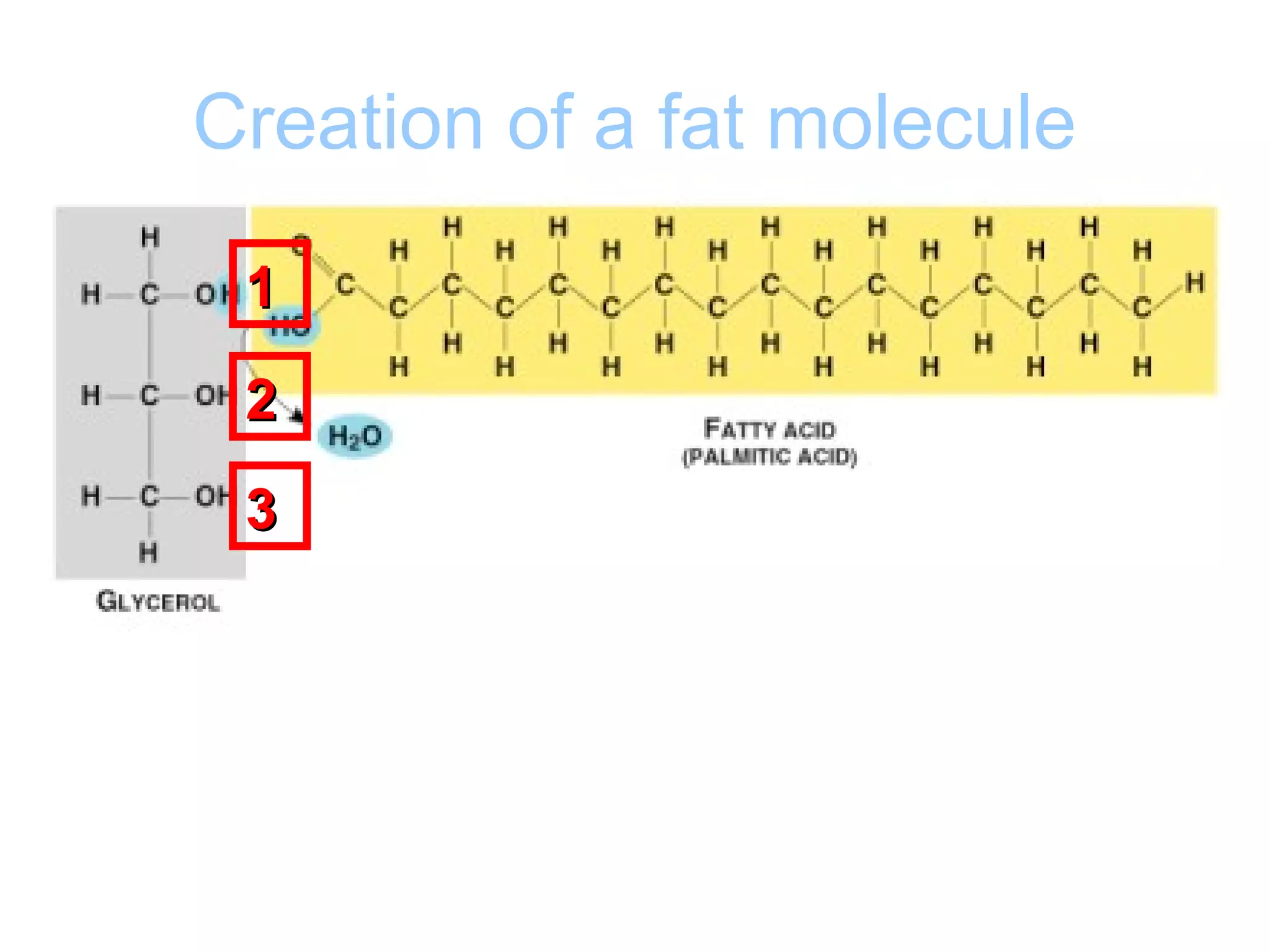
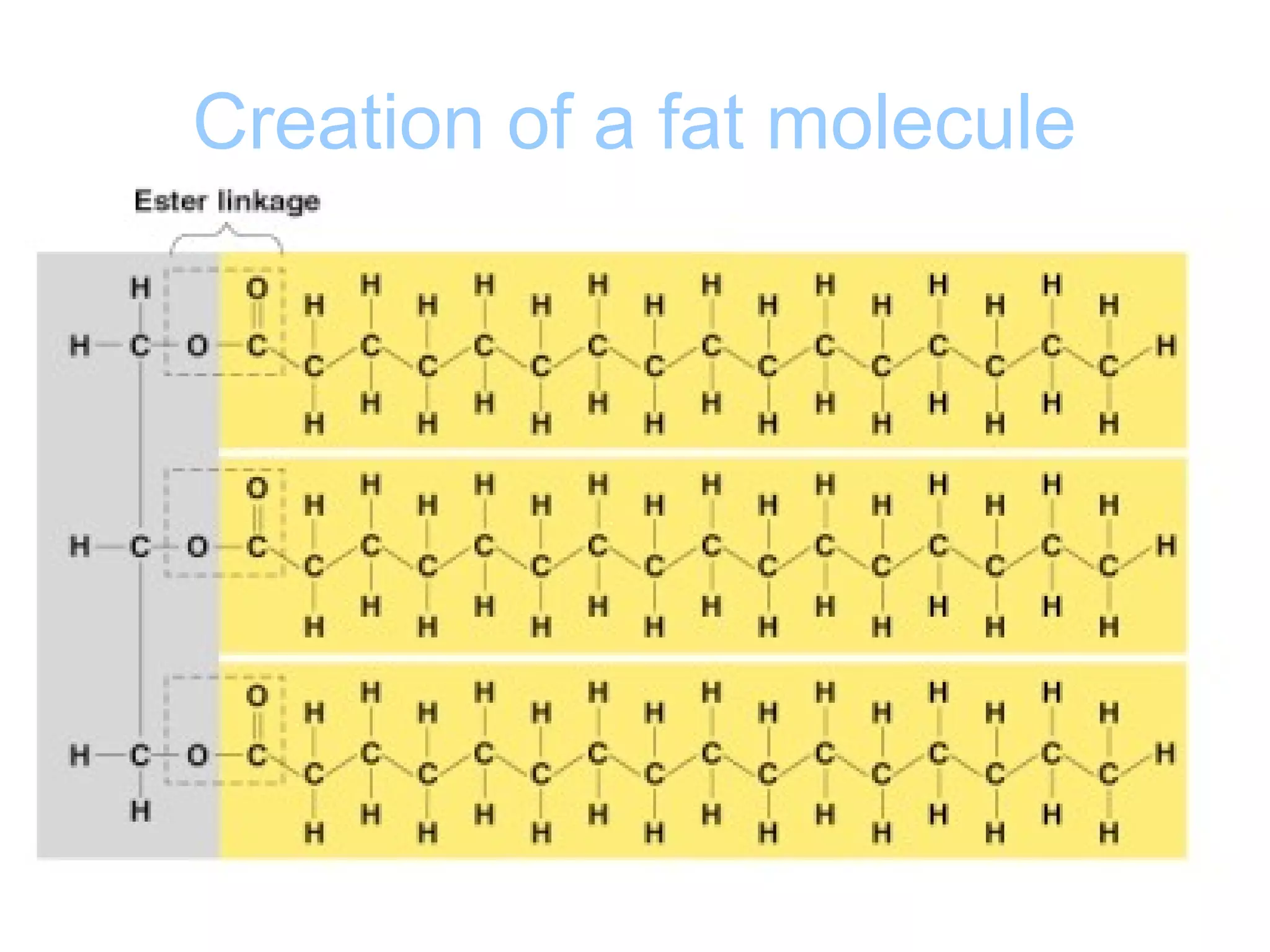
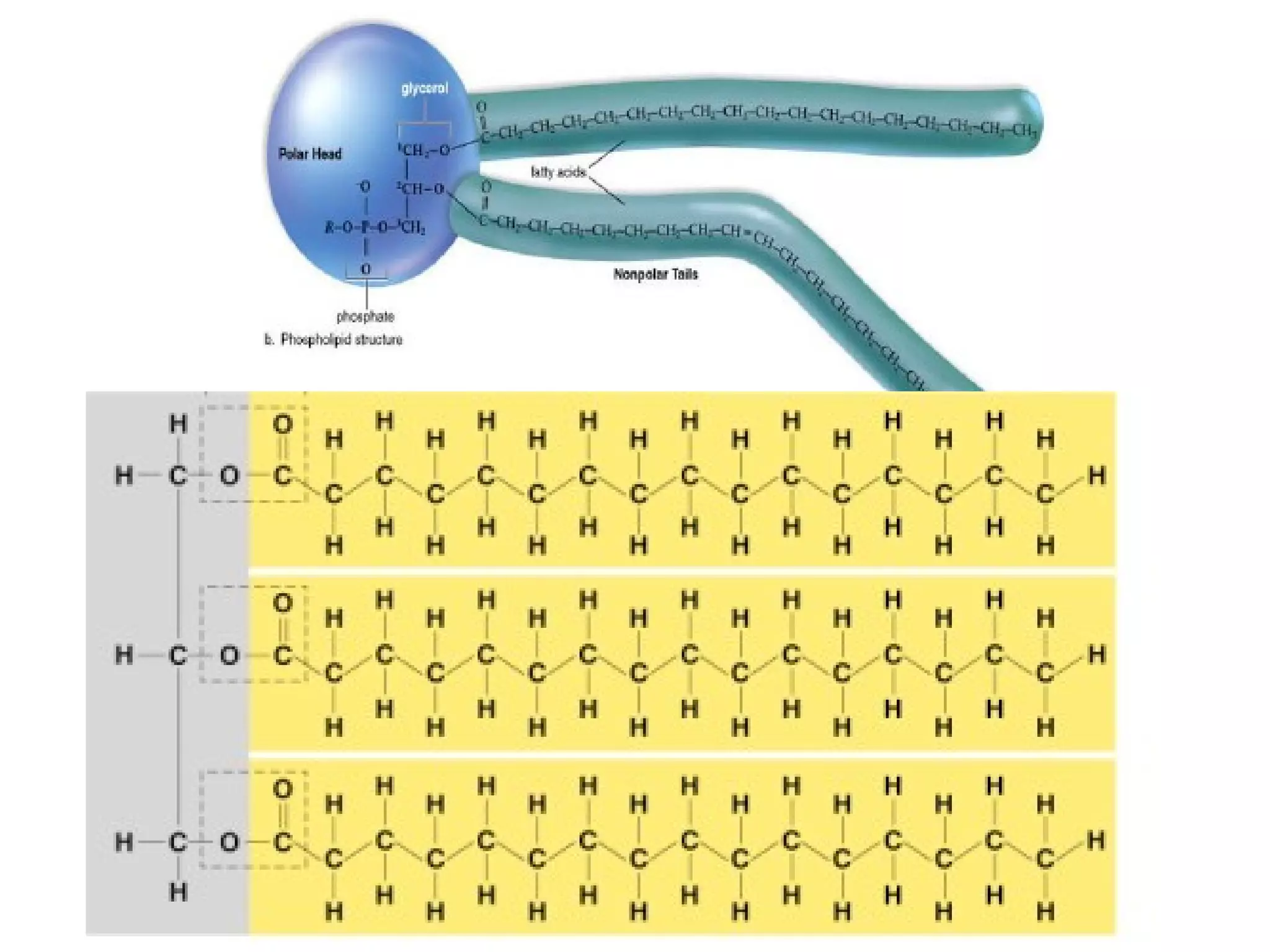
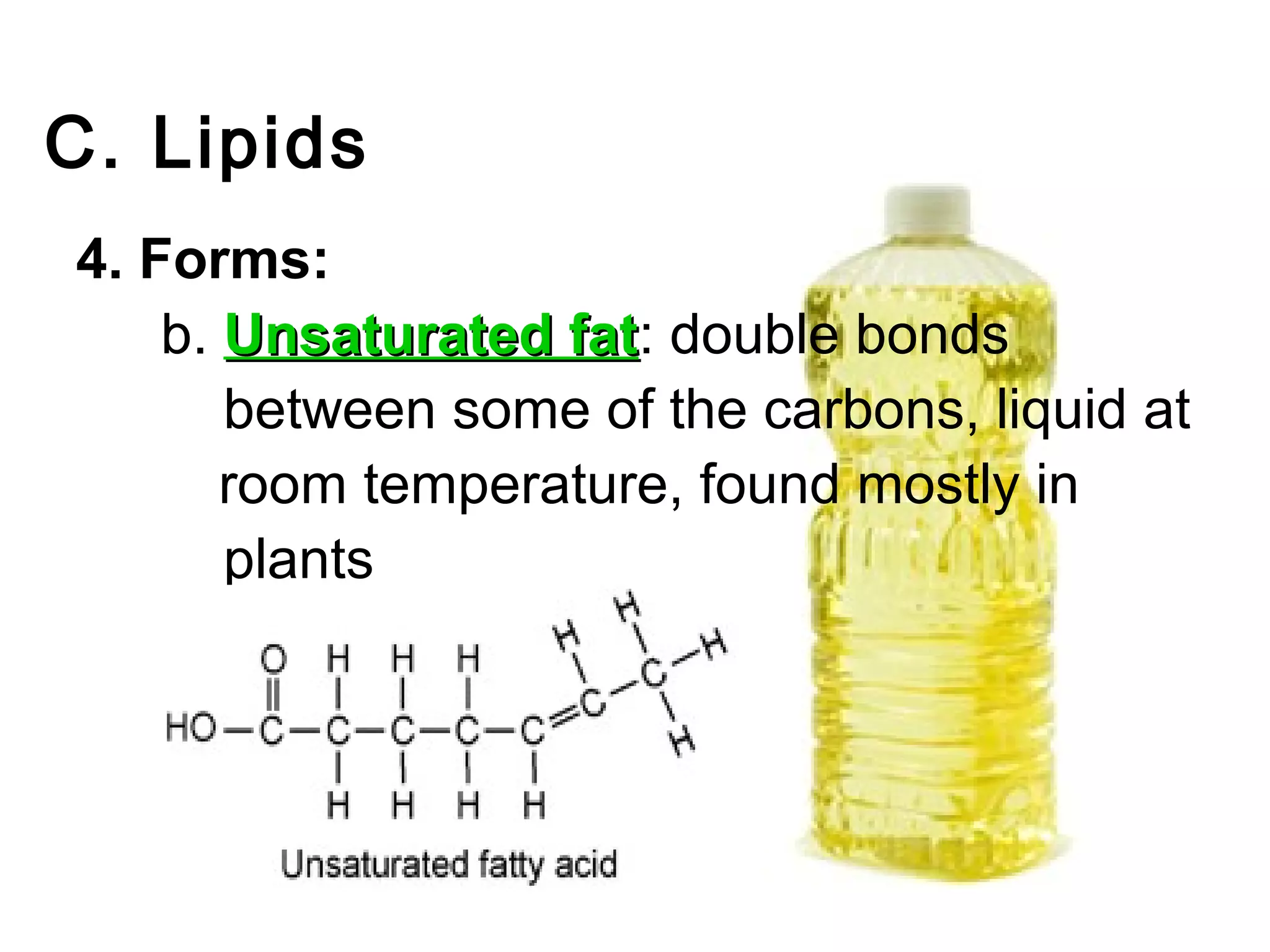
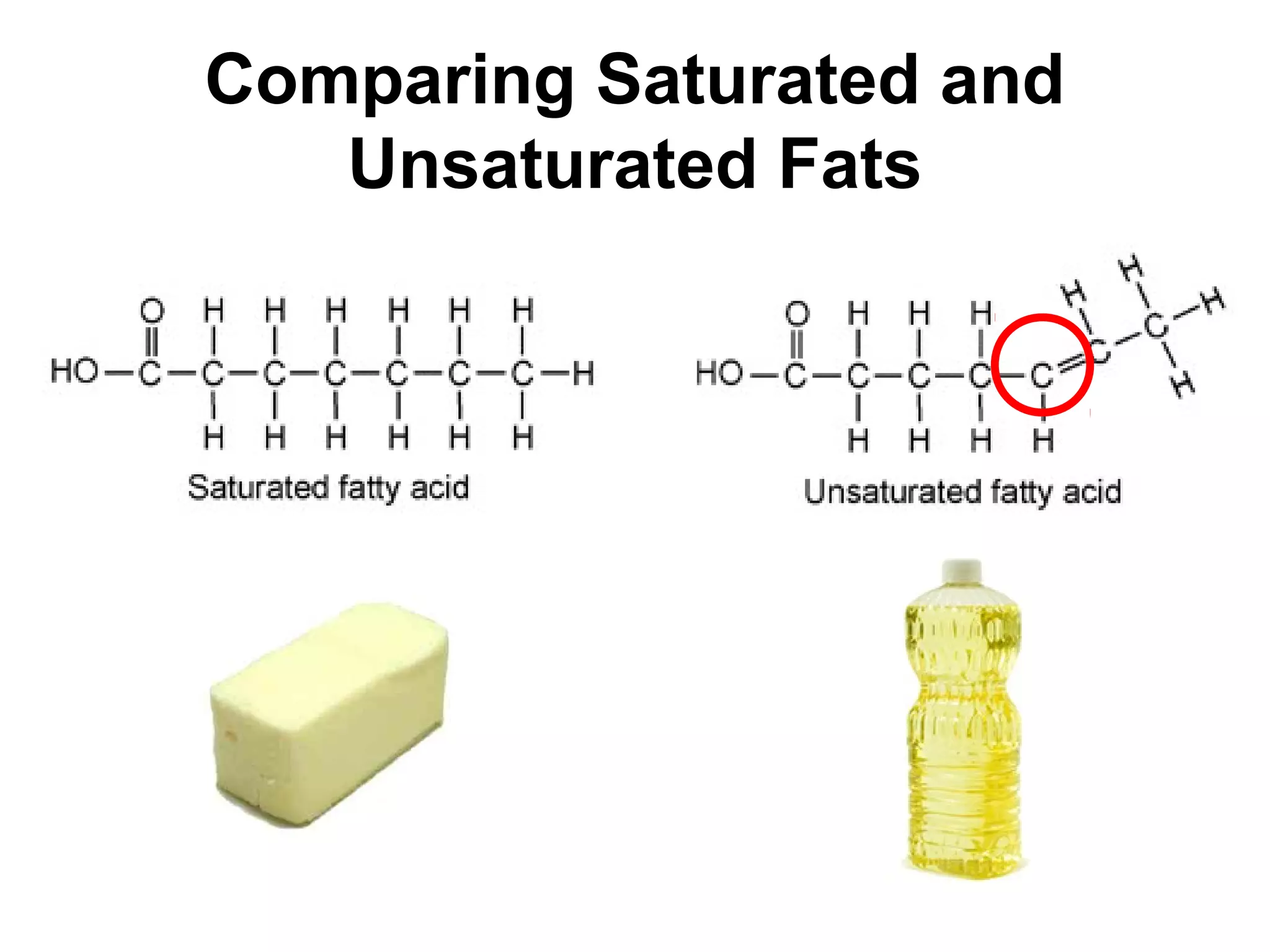
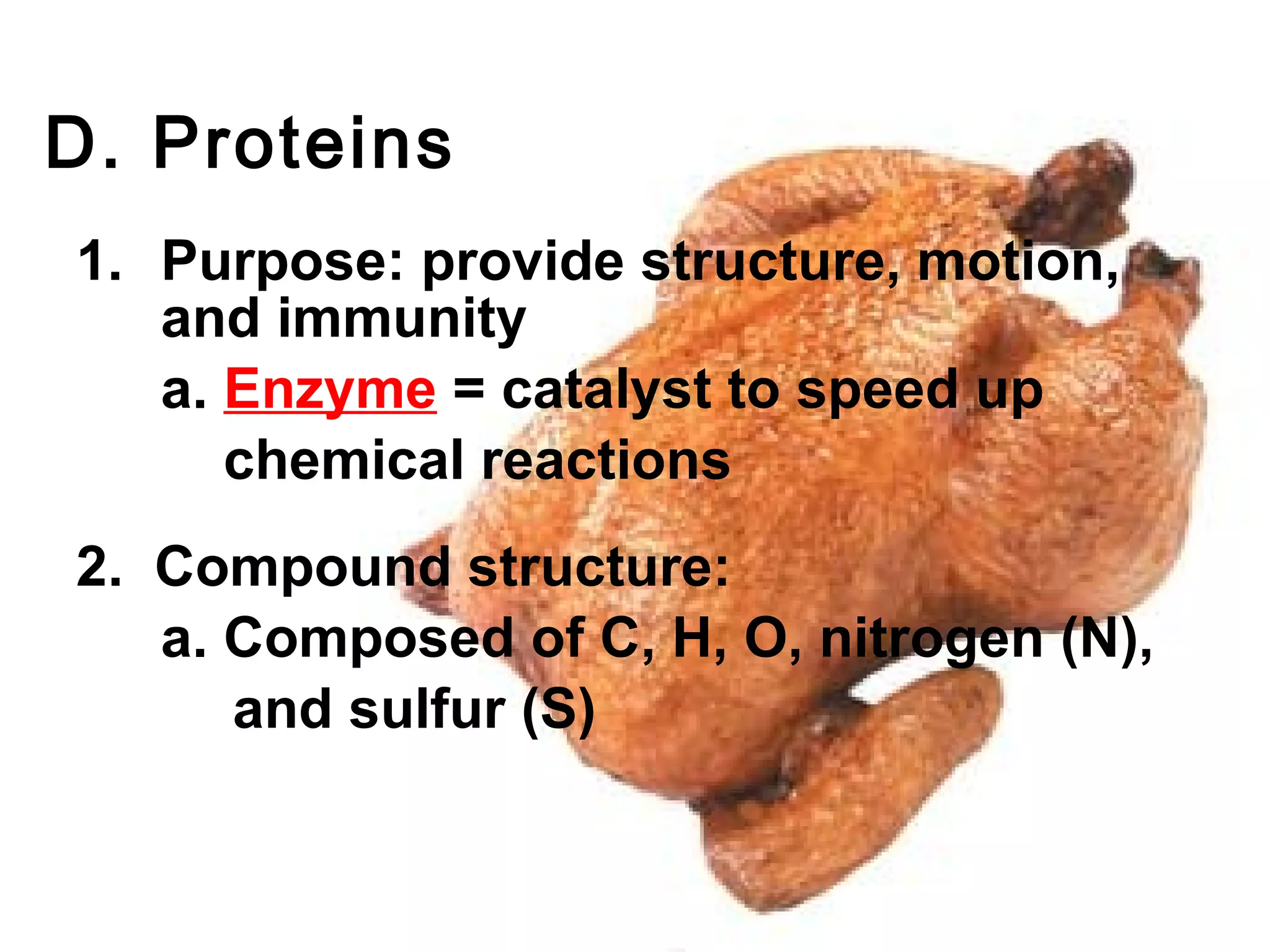
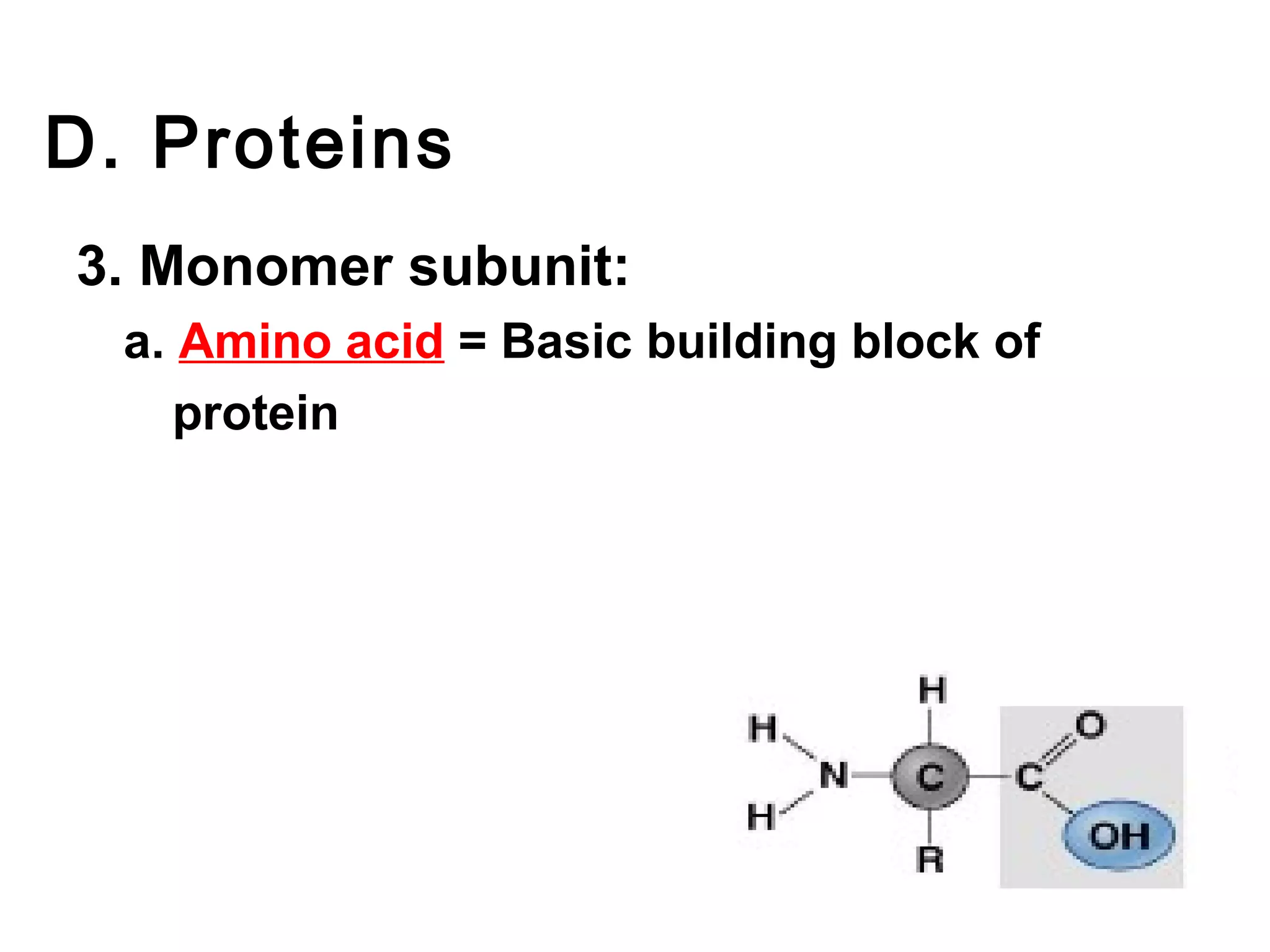
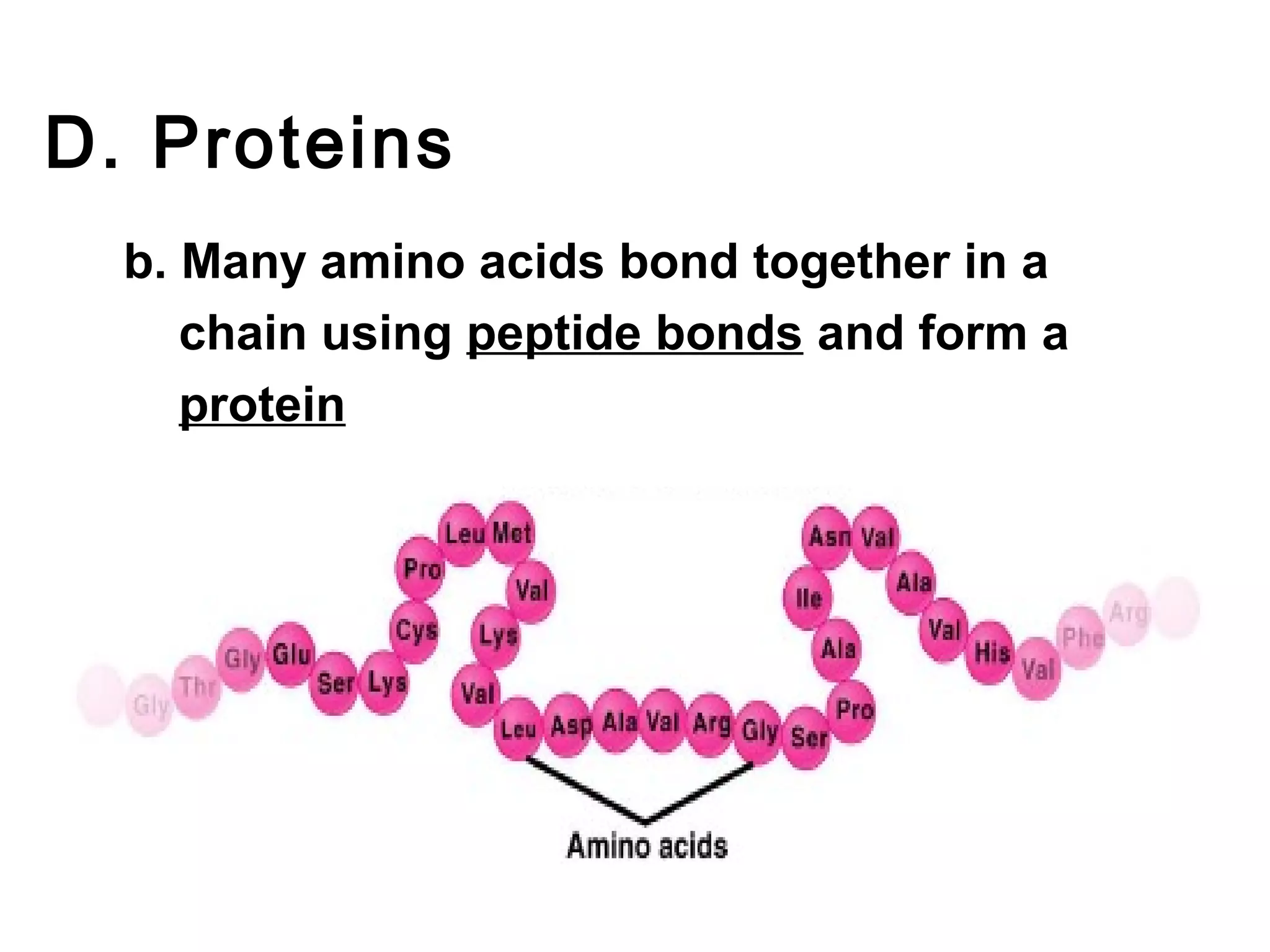
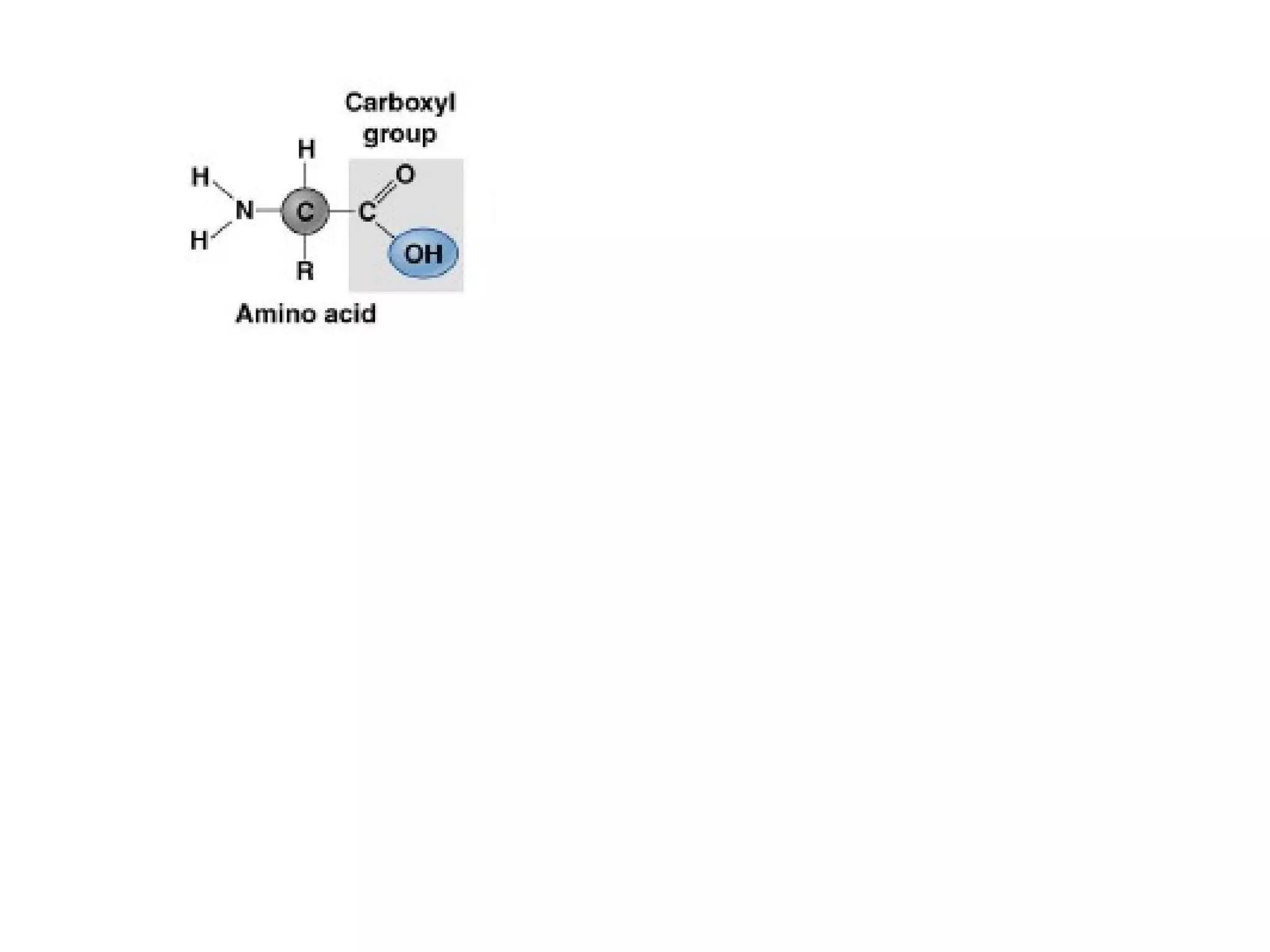
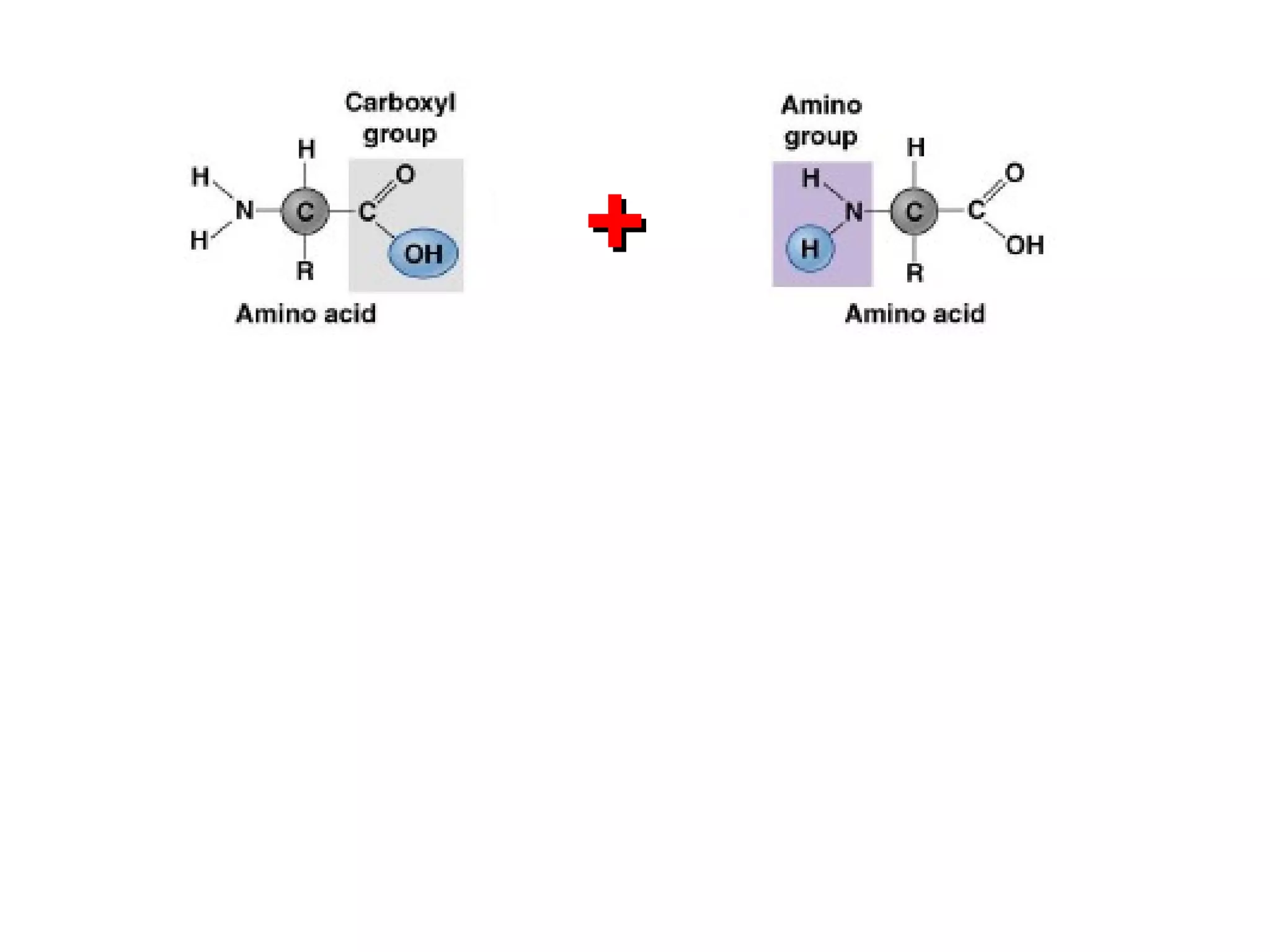
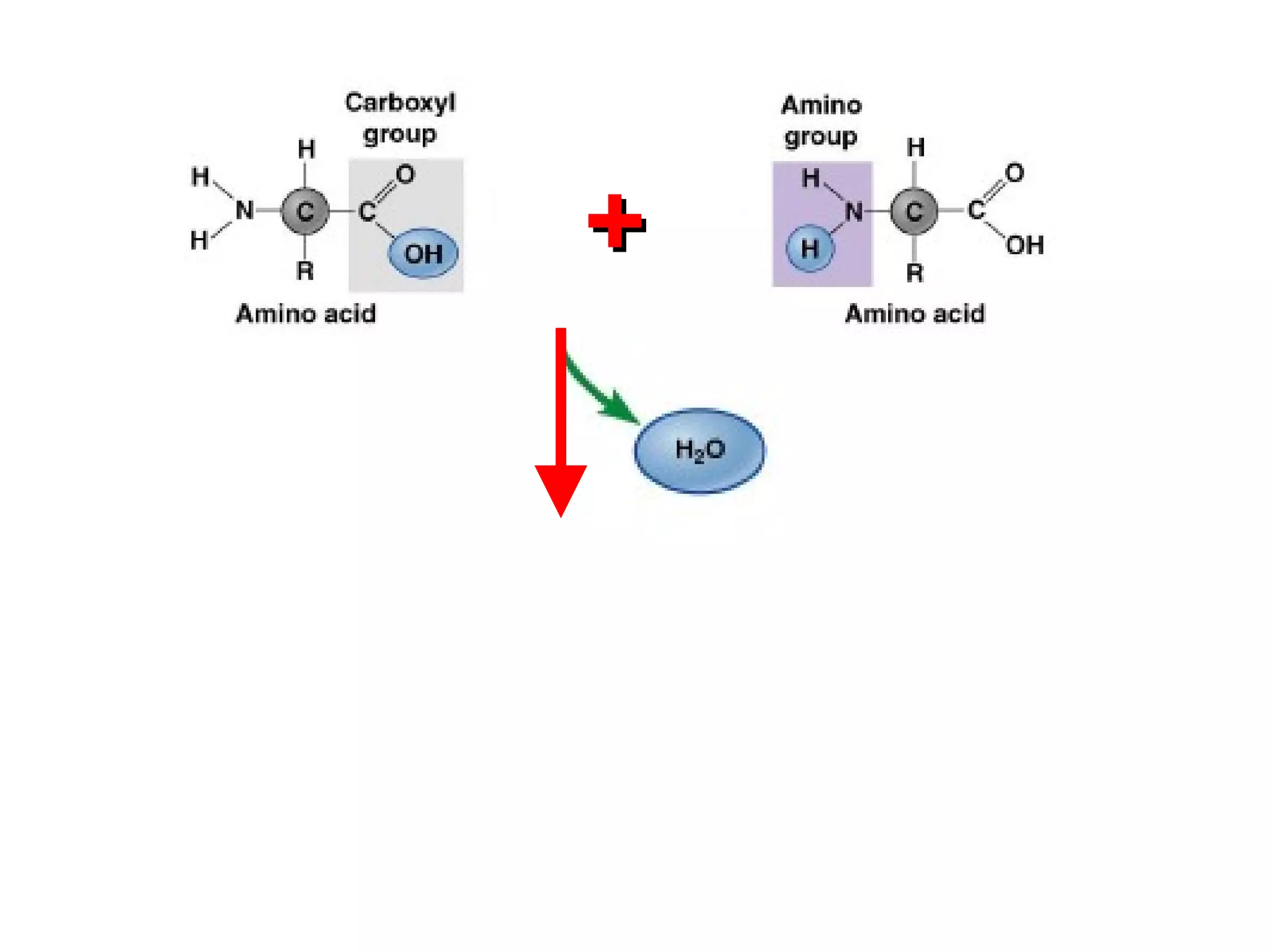
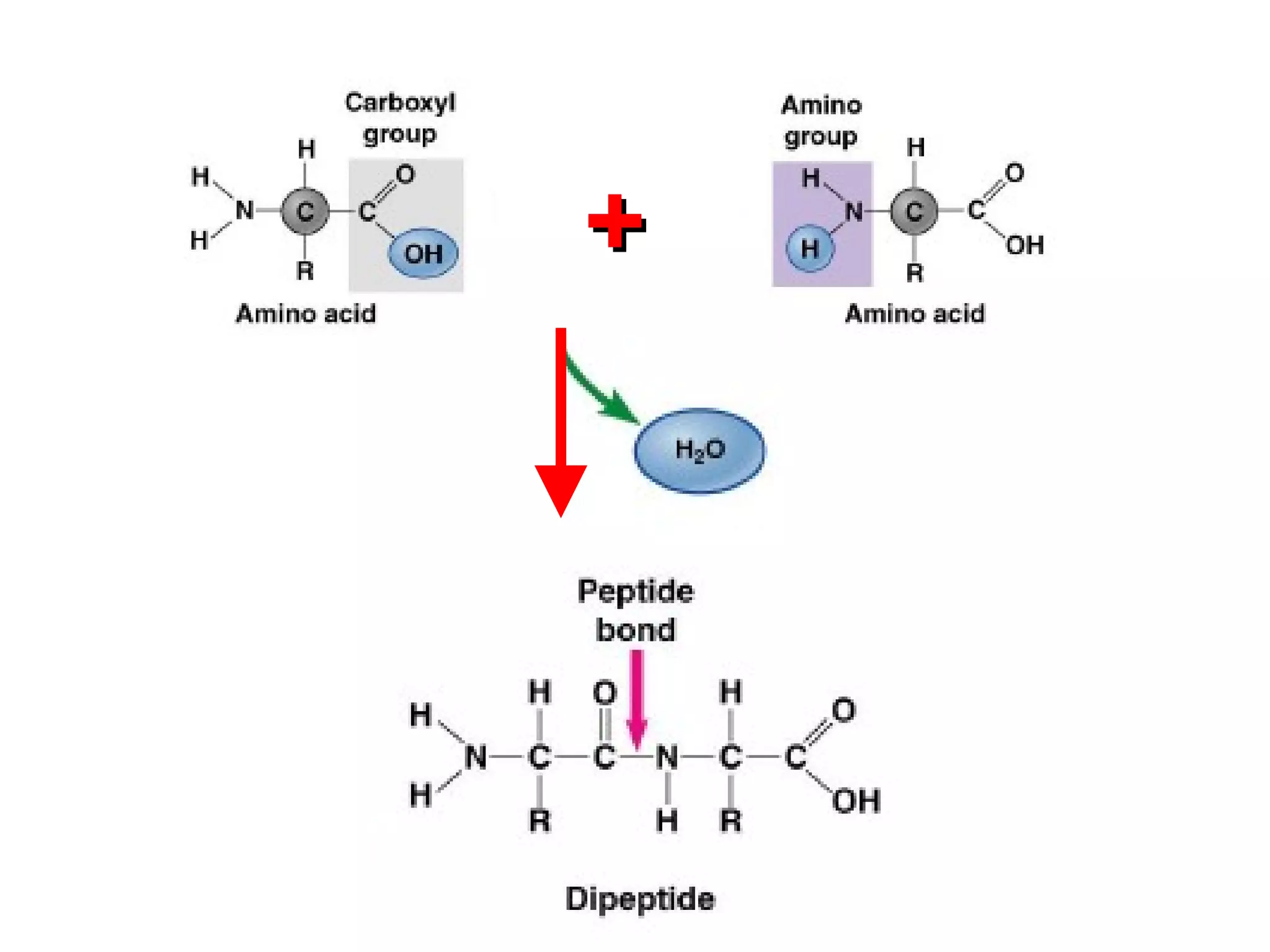
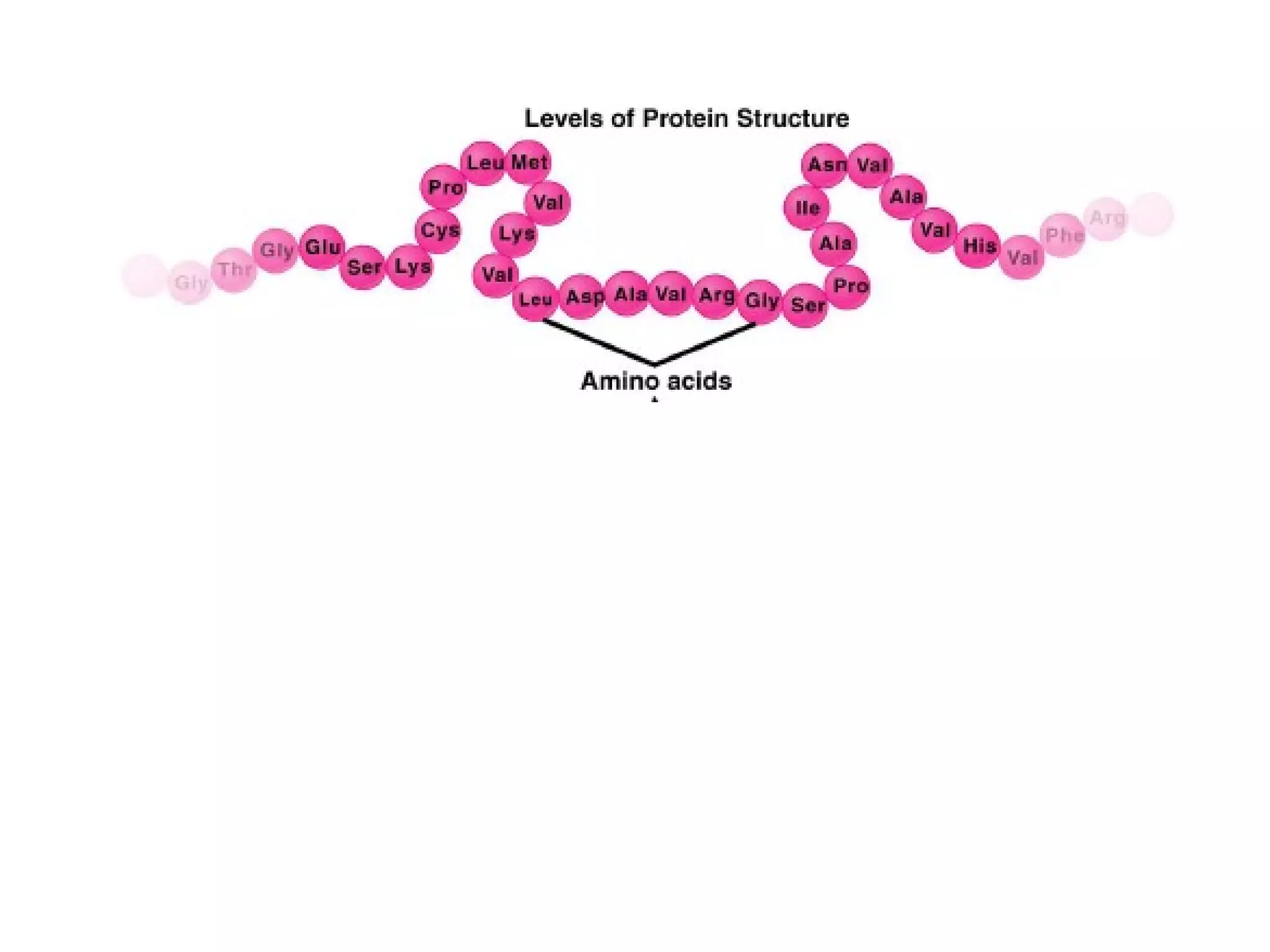
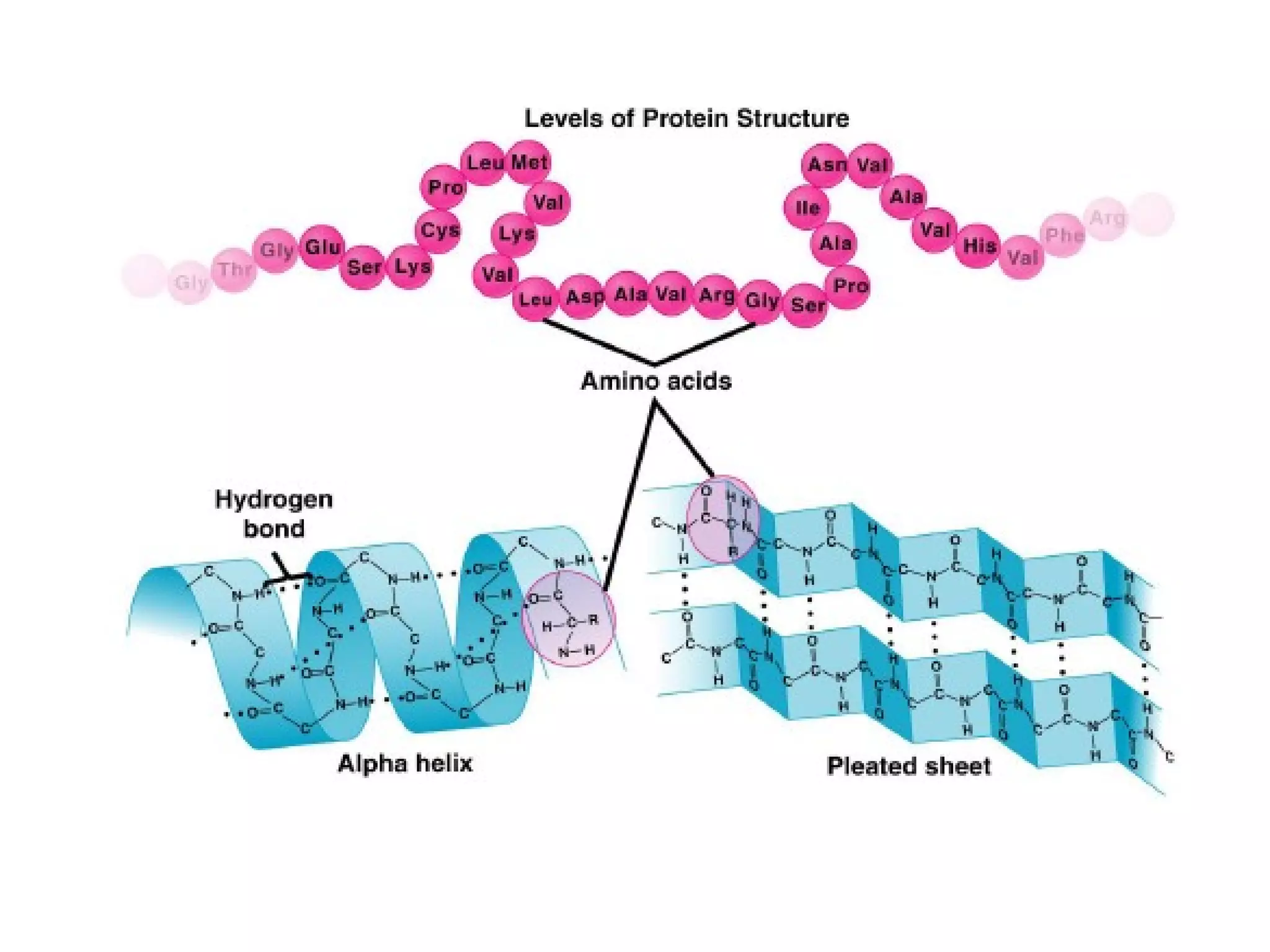
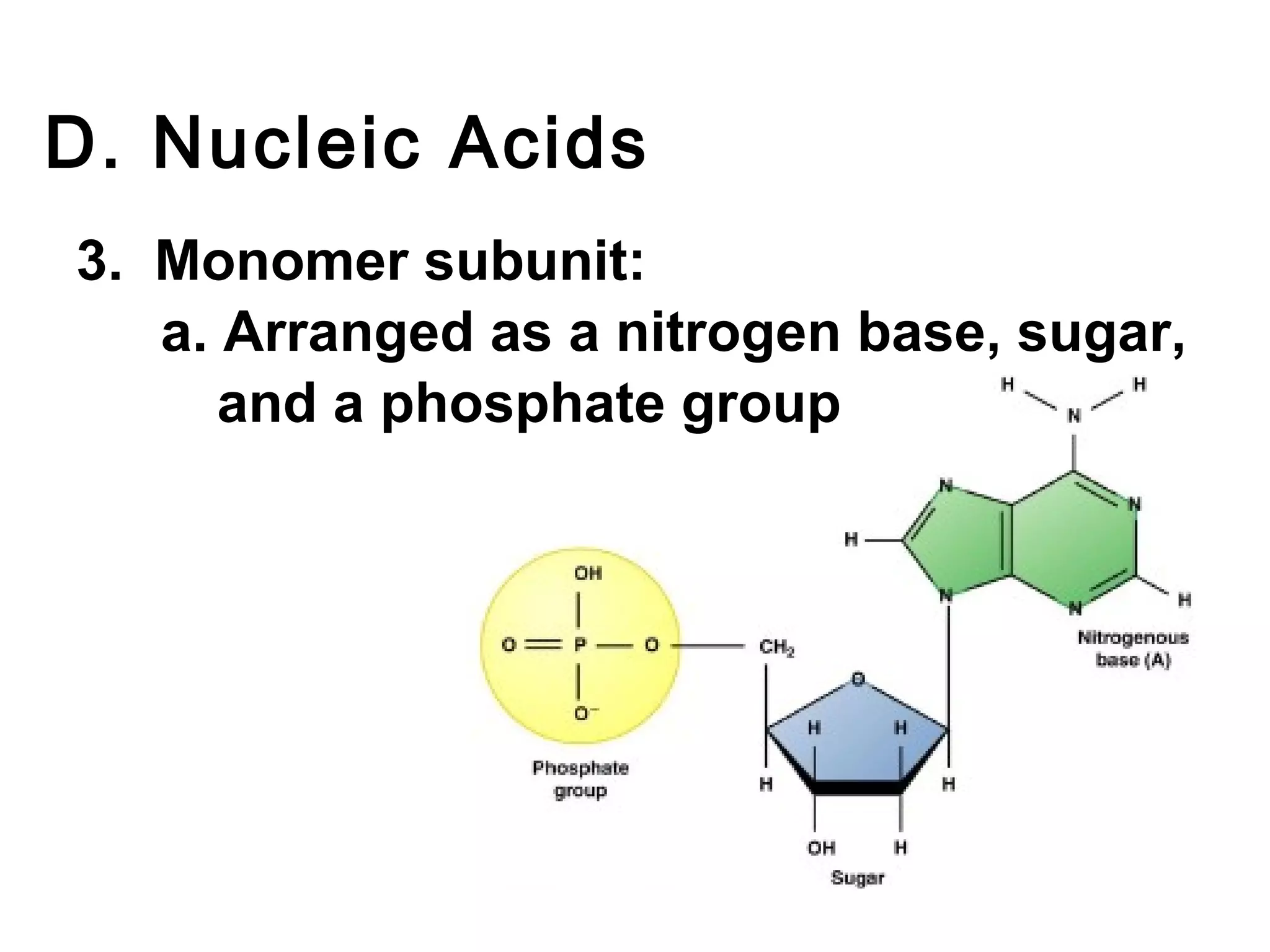
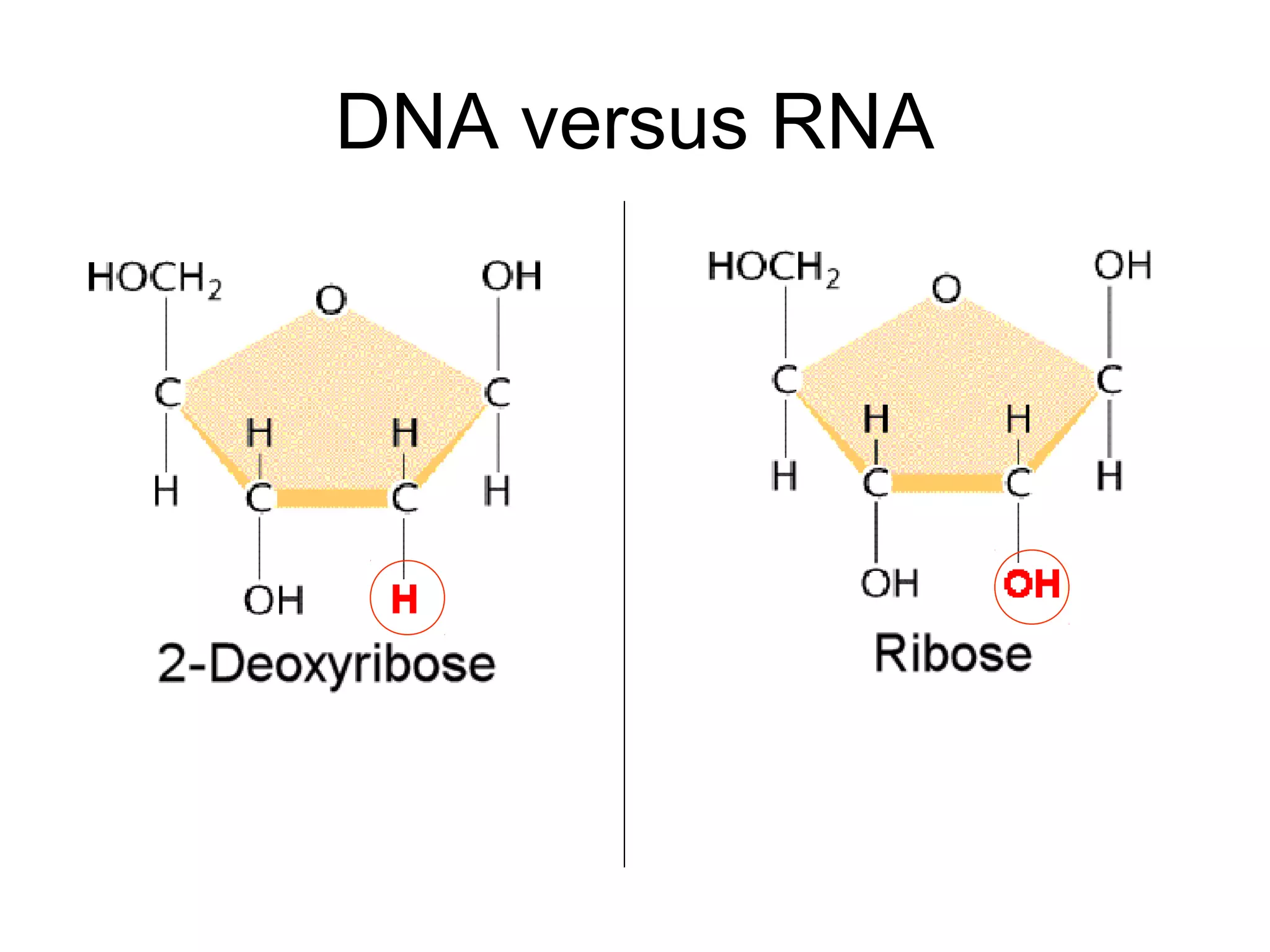
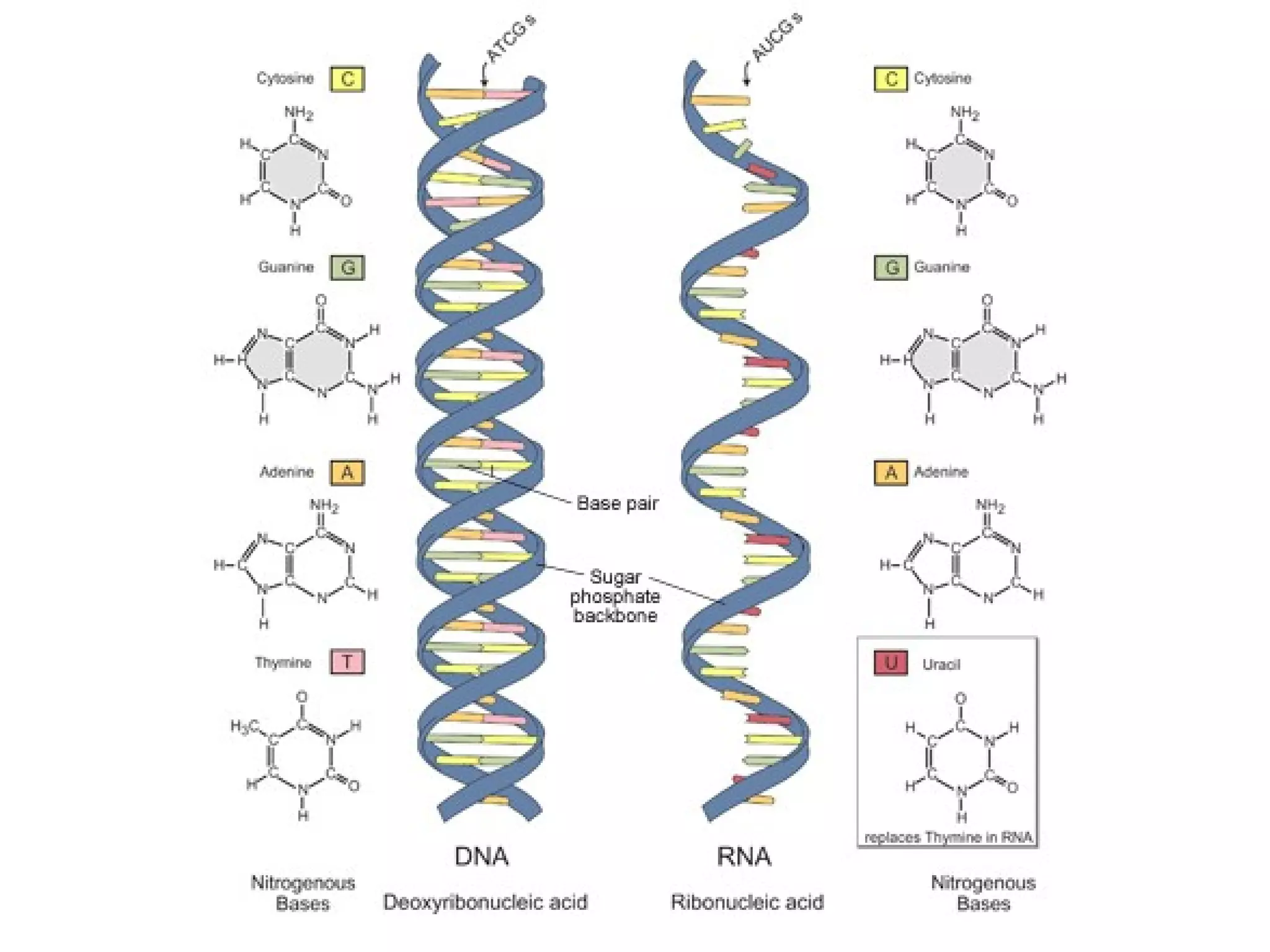
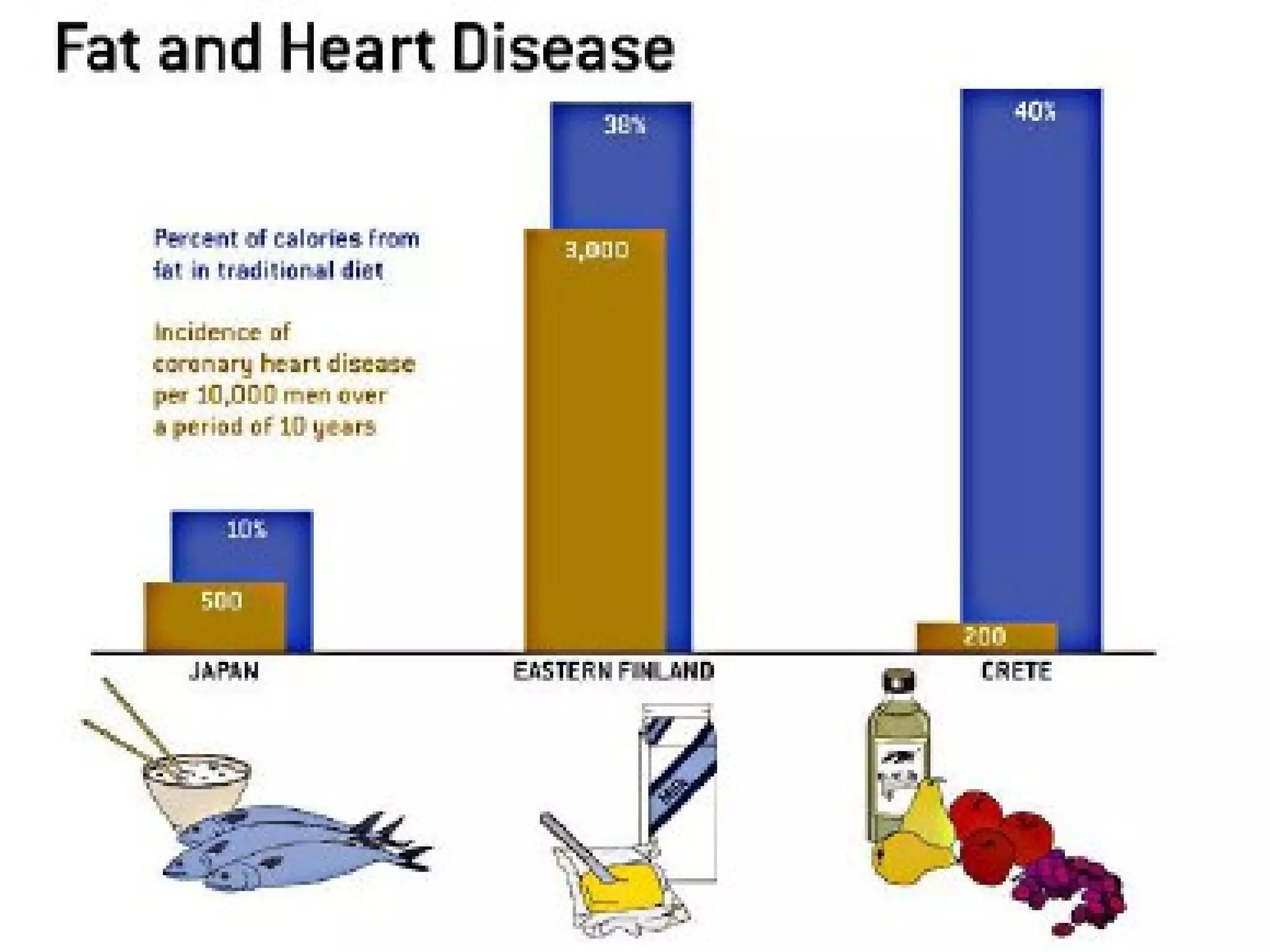
The document provides an outline on biochemistry and organic biomolecules. It begins by listing learning targets about the elements that make up living things and the structures of carbohydrates, lipids, proteins, and nucleic acids. It then discusses atoms and their interactions, including atomic structure and how atoms bond to form molecules. The four main types of organic biomolecules - carbohydrates, lipids, proteins, and nucleic acids - are then described in detail, including their purposes, structures, monomer subunits, and examples.