Embed presentation
Downloaded 458 times

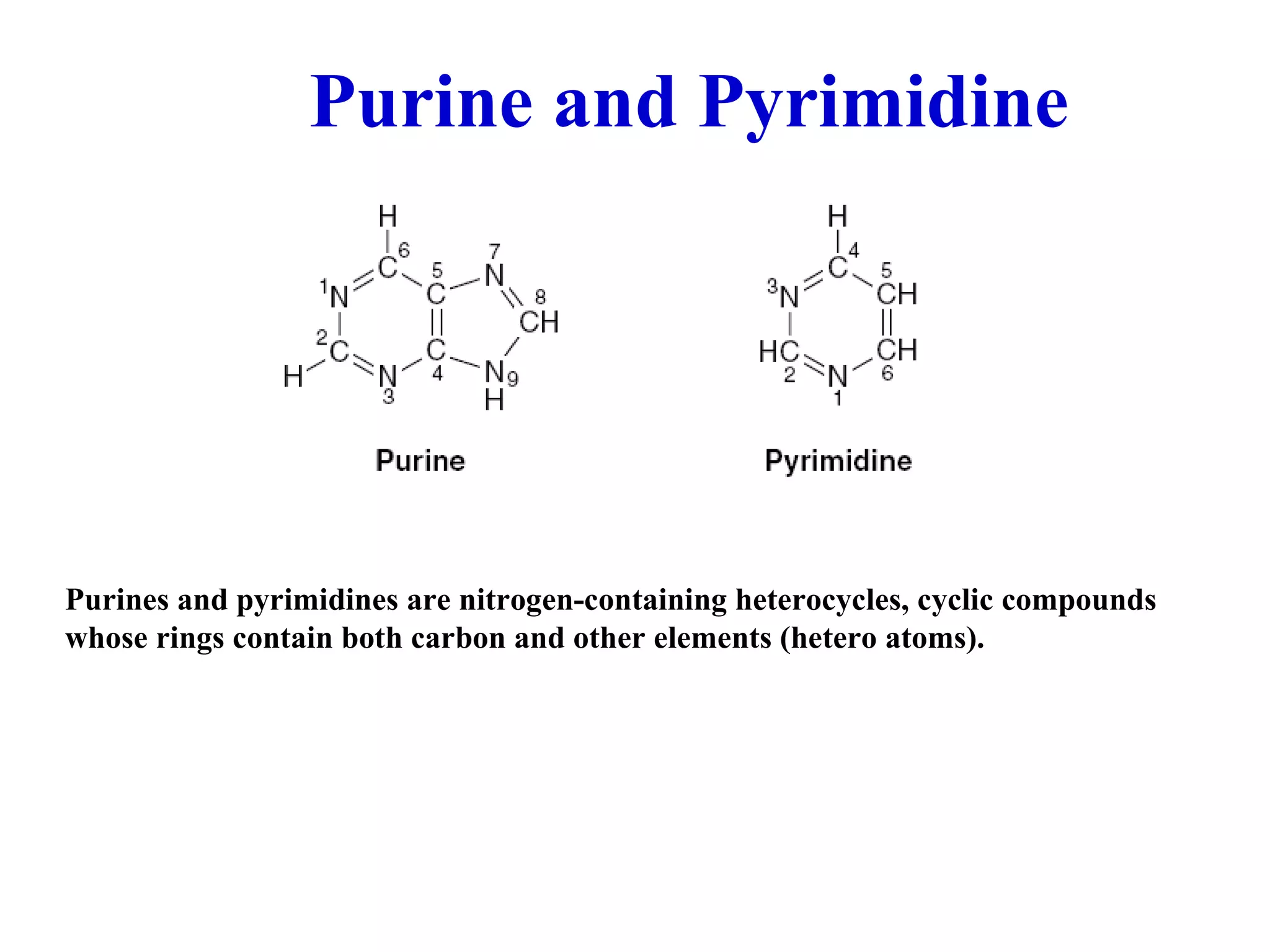
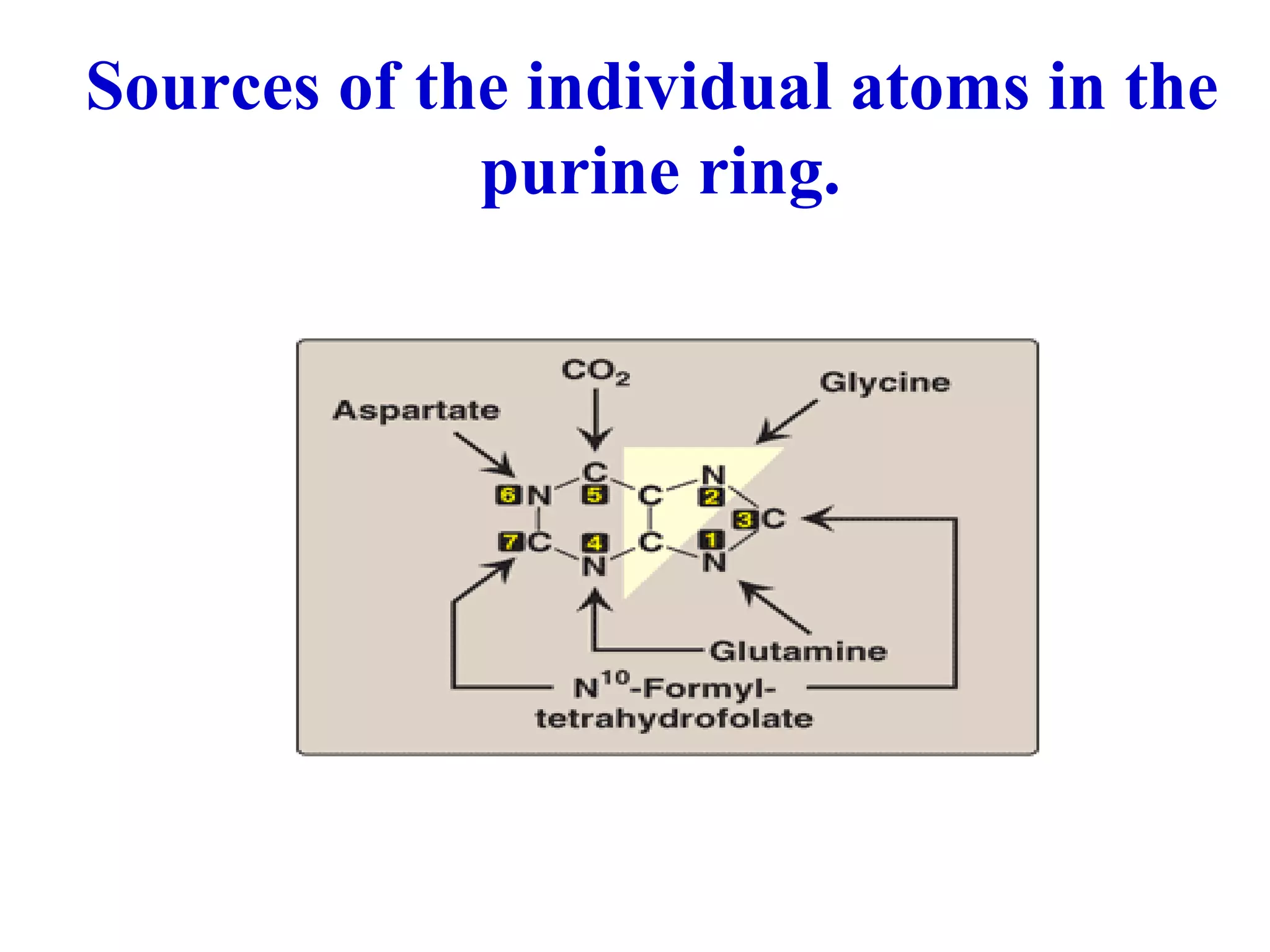
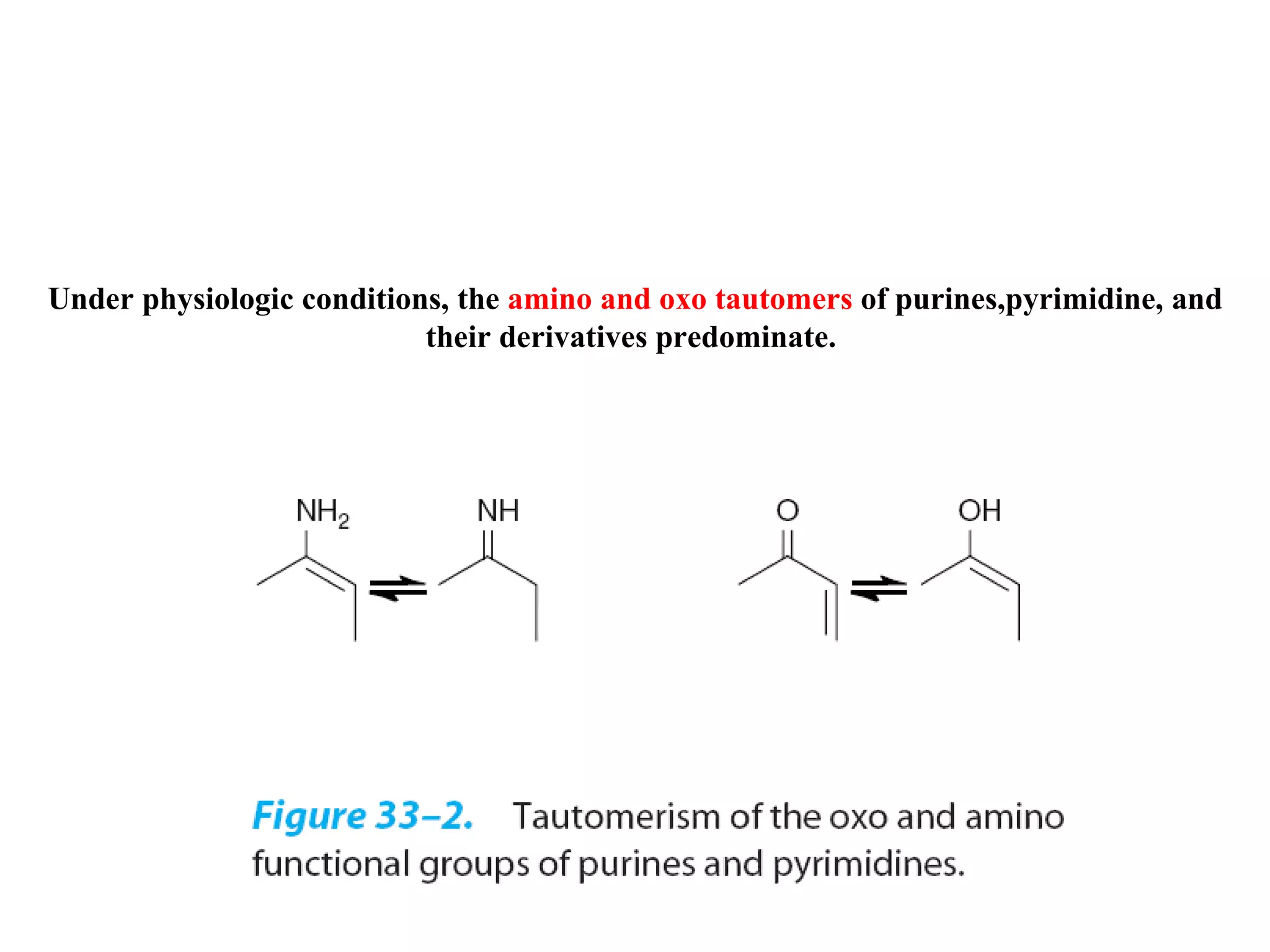
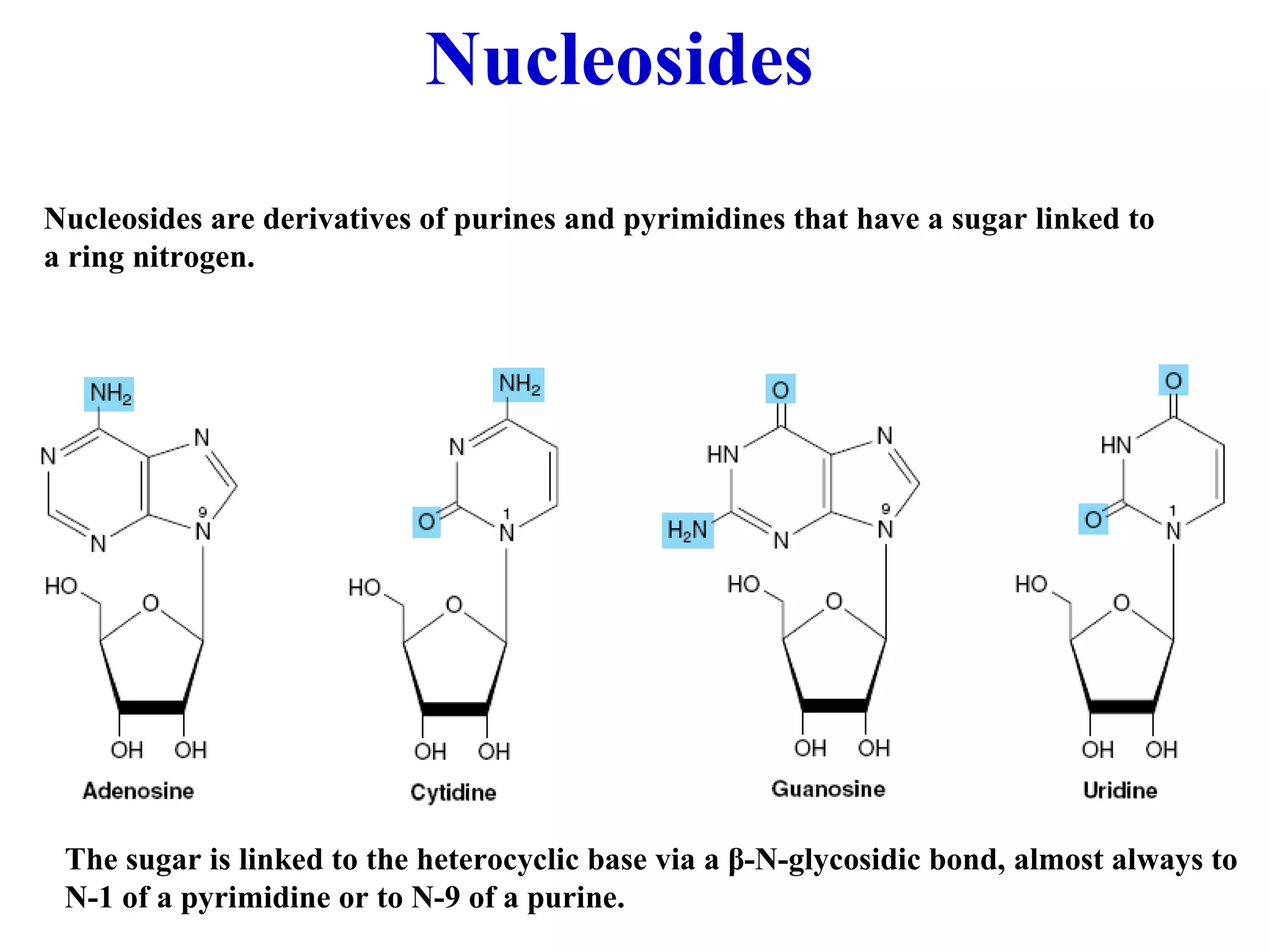
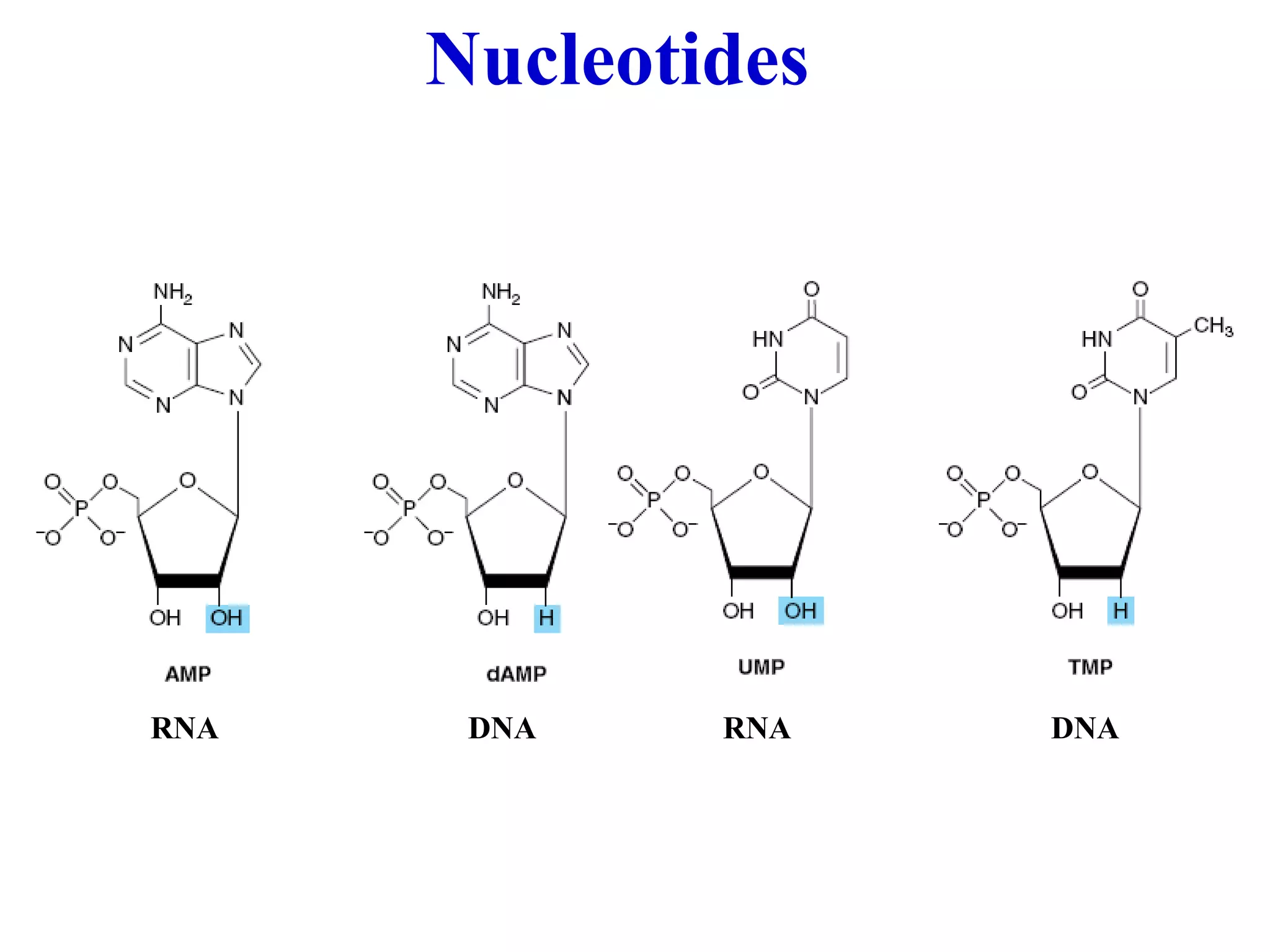
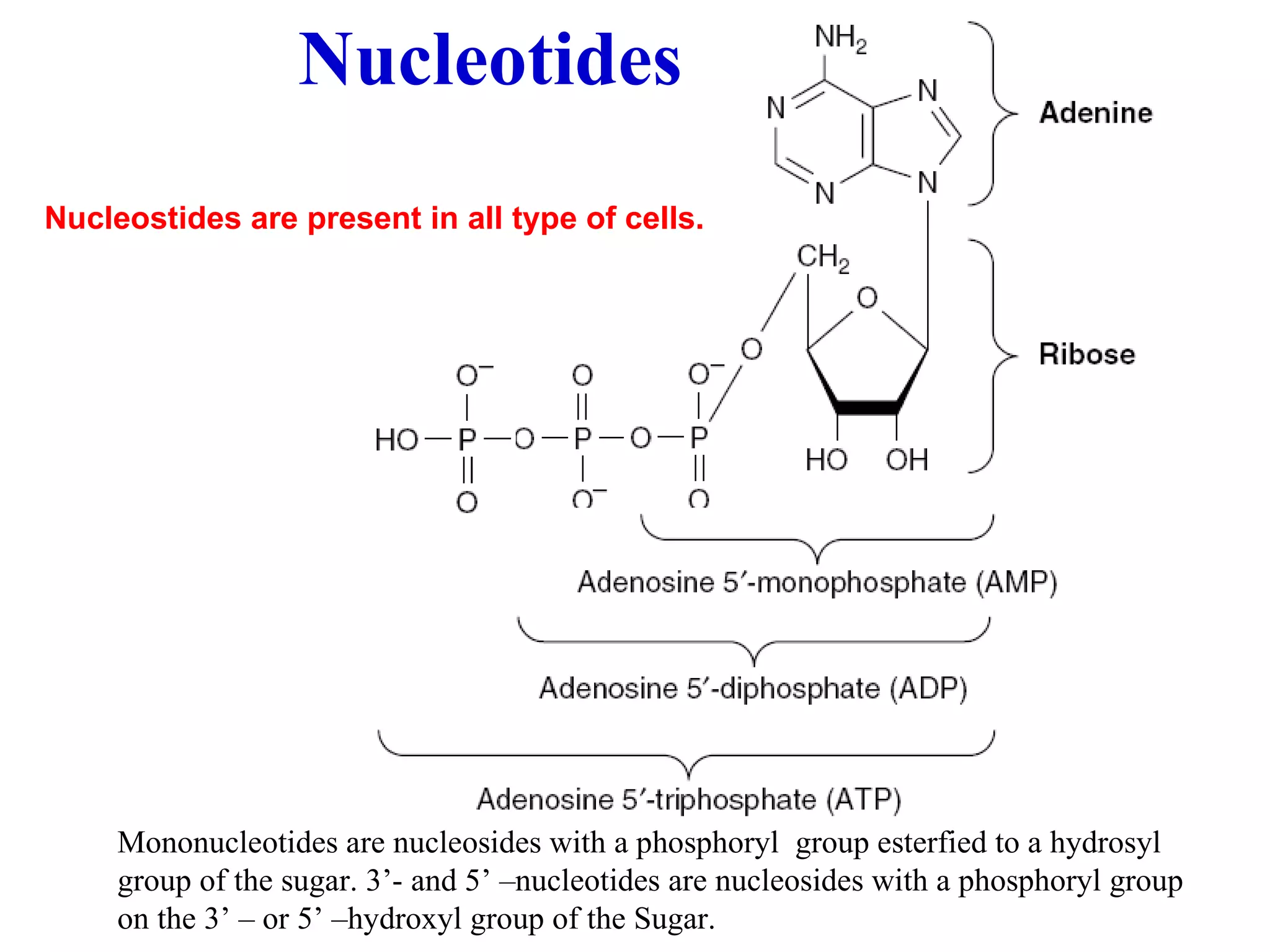
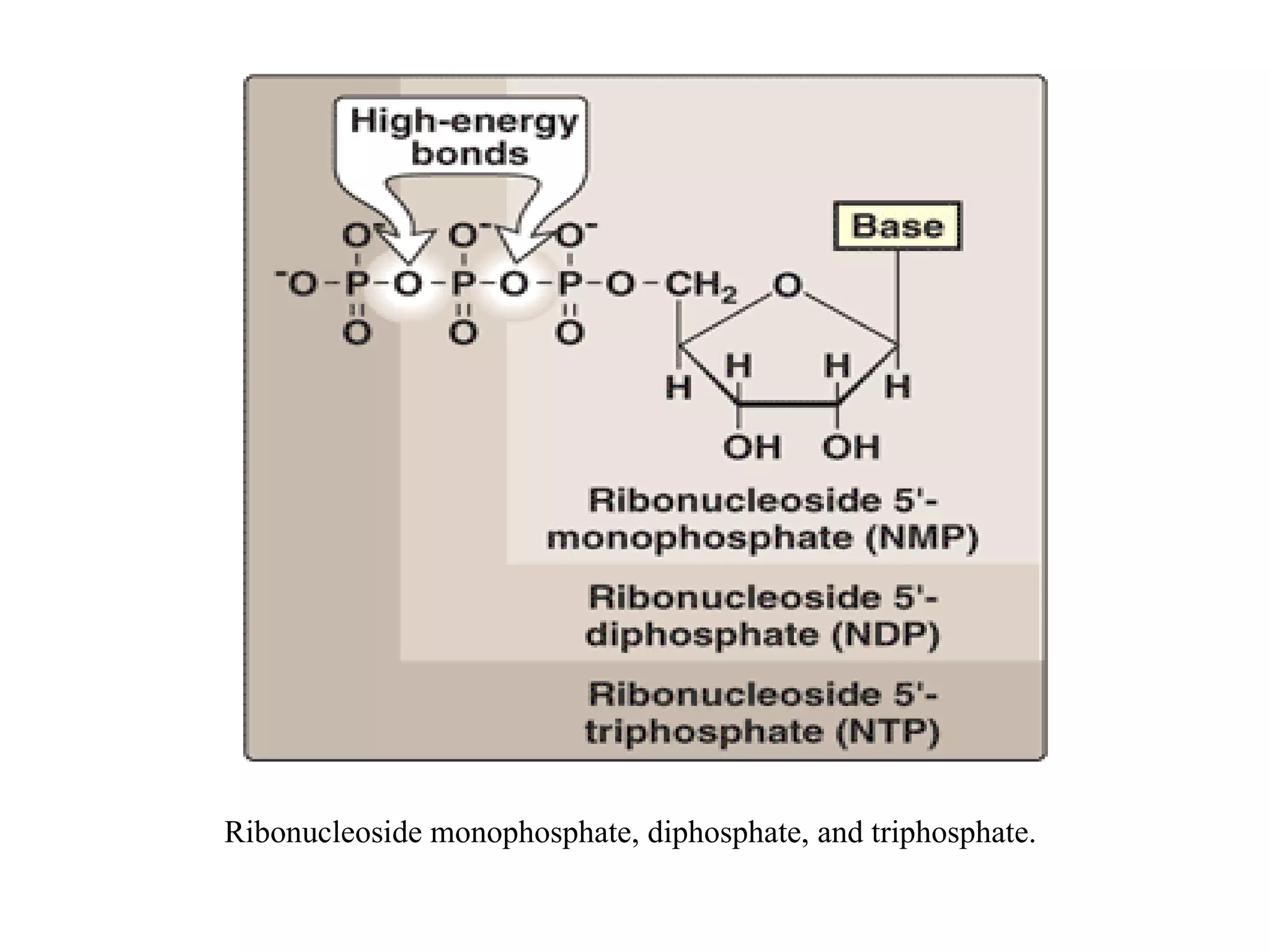
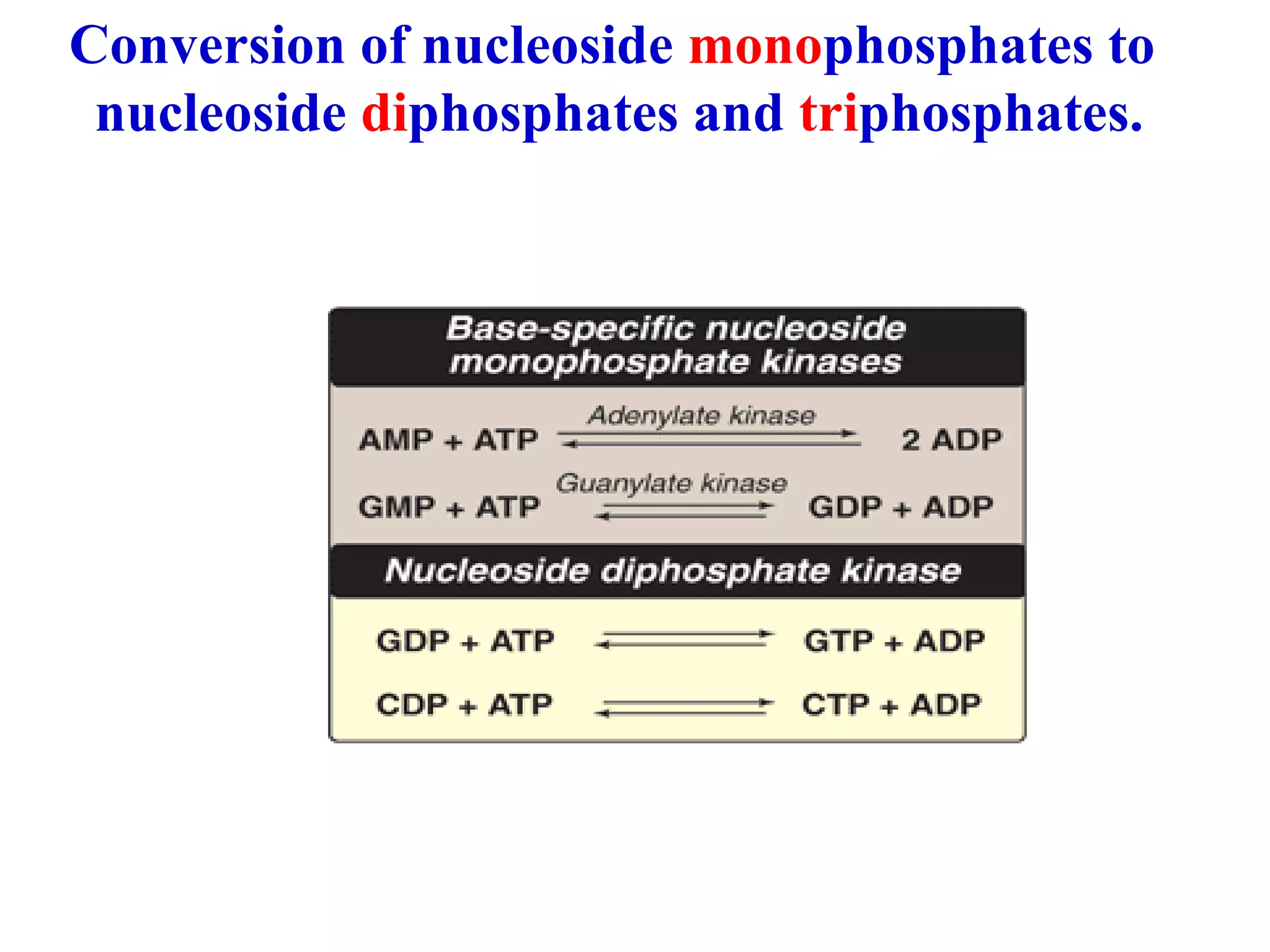
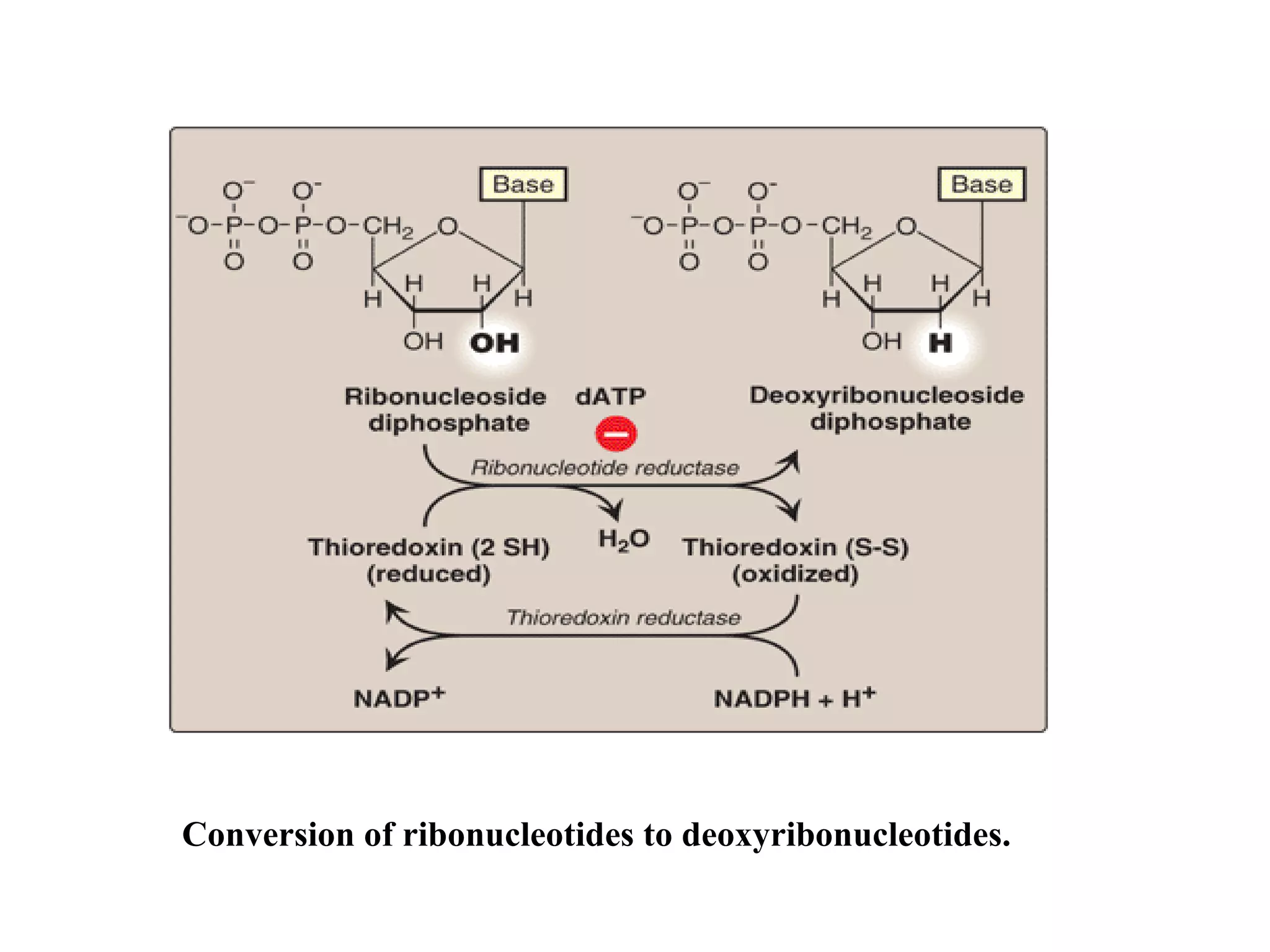
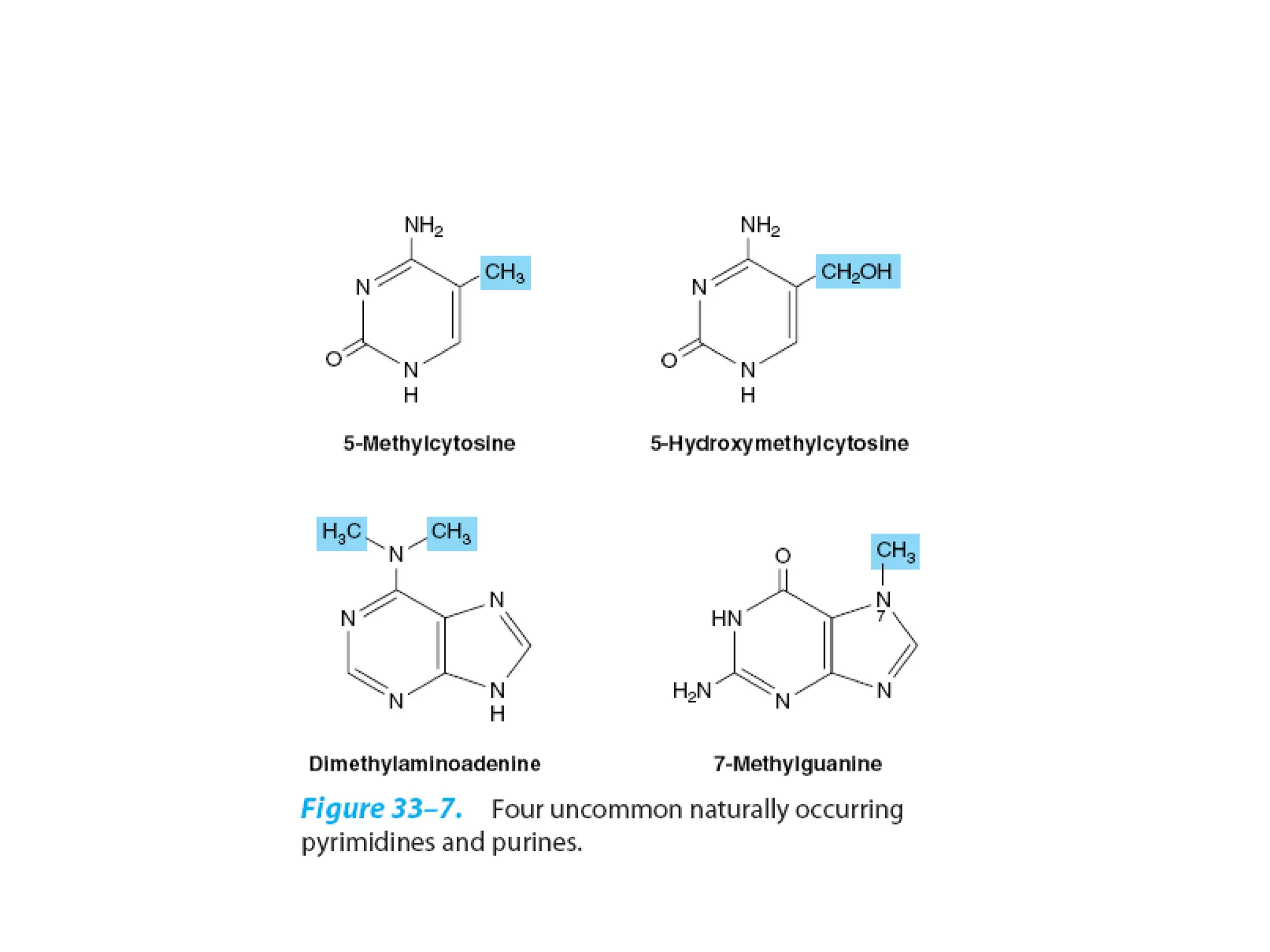
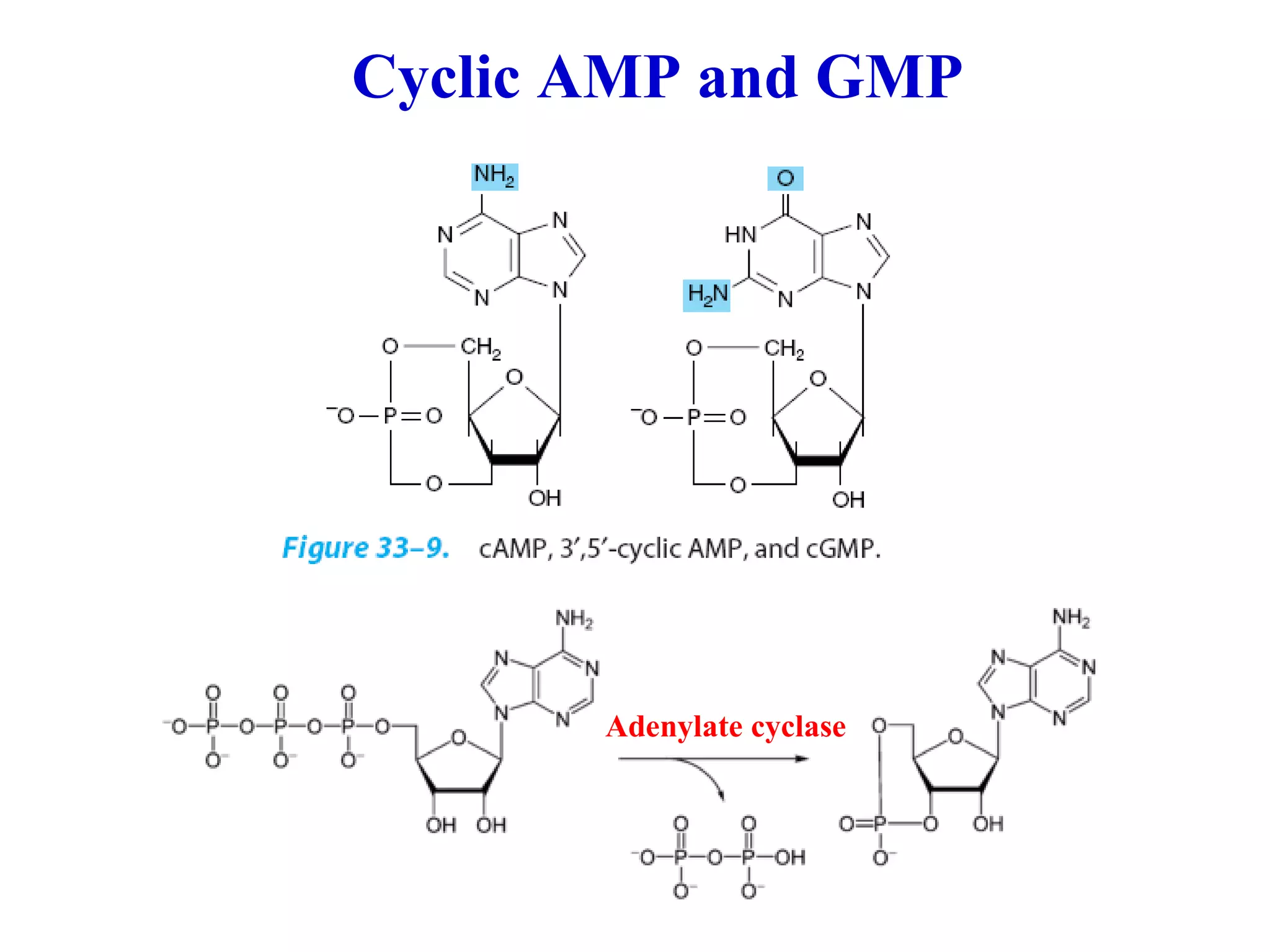
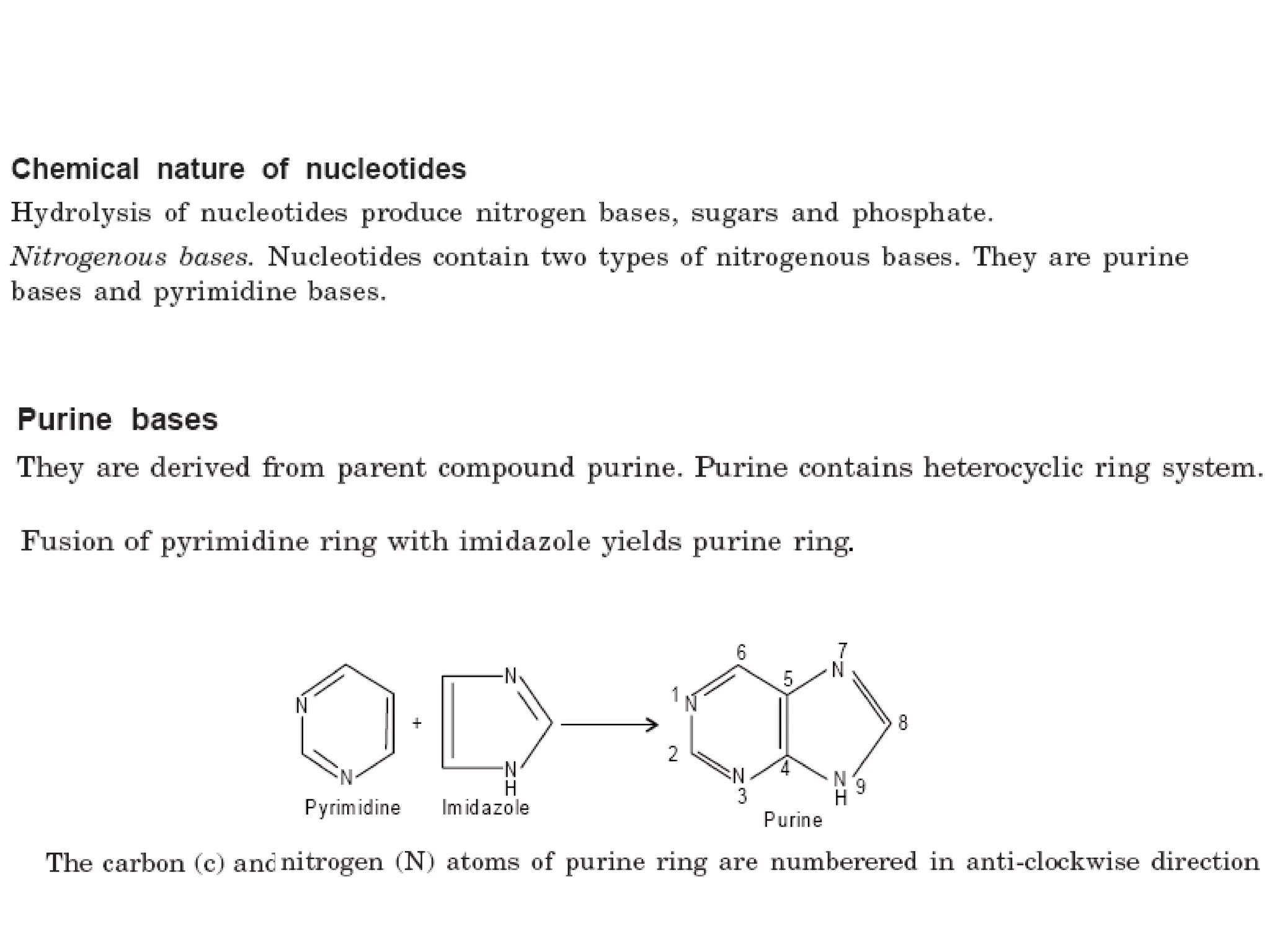
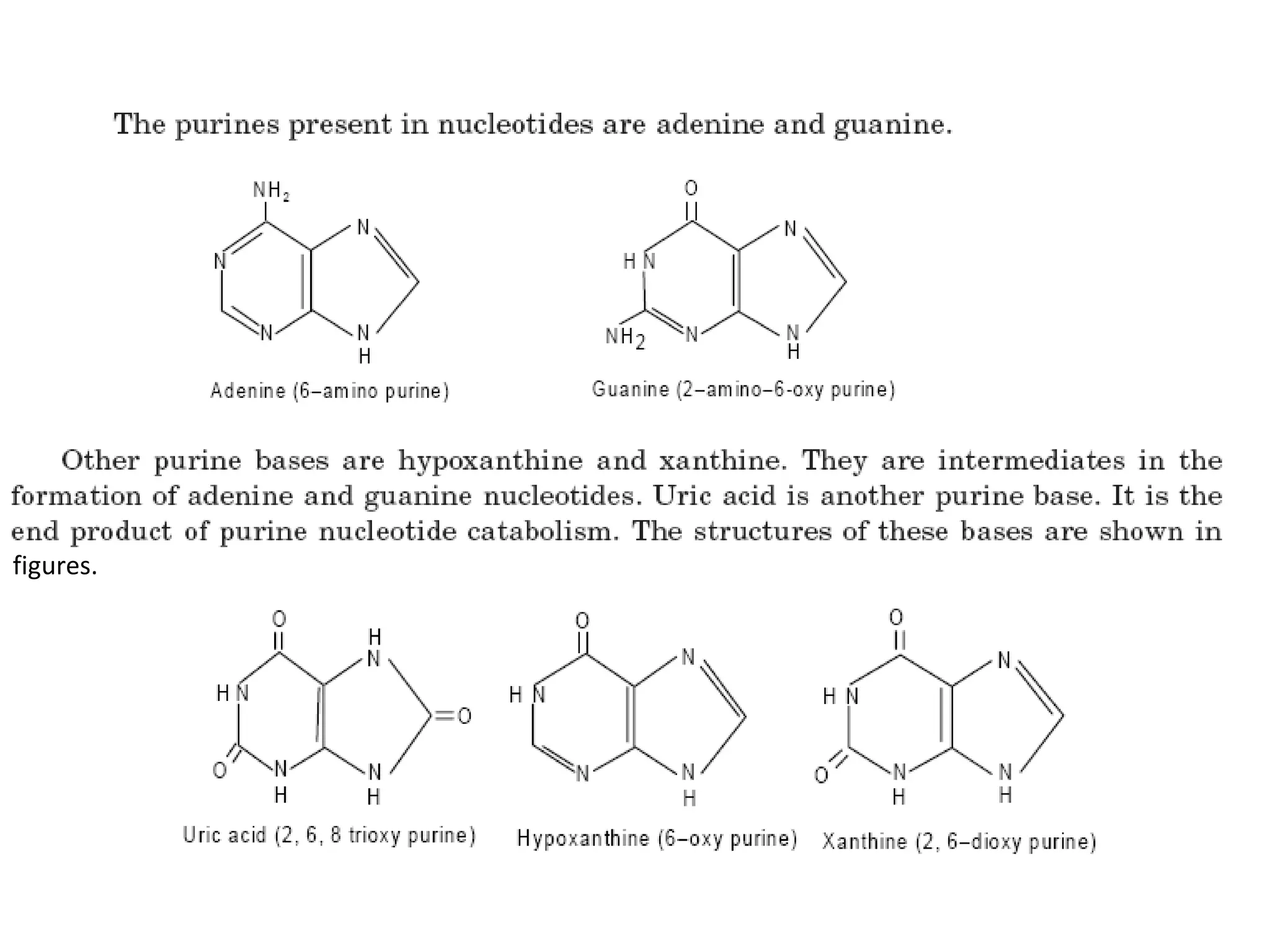
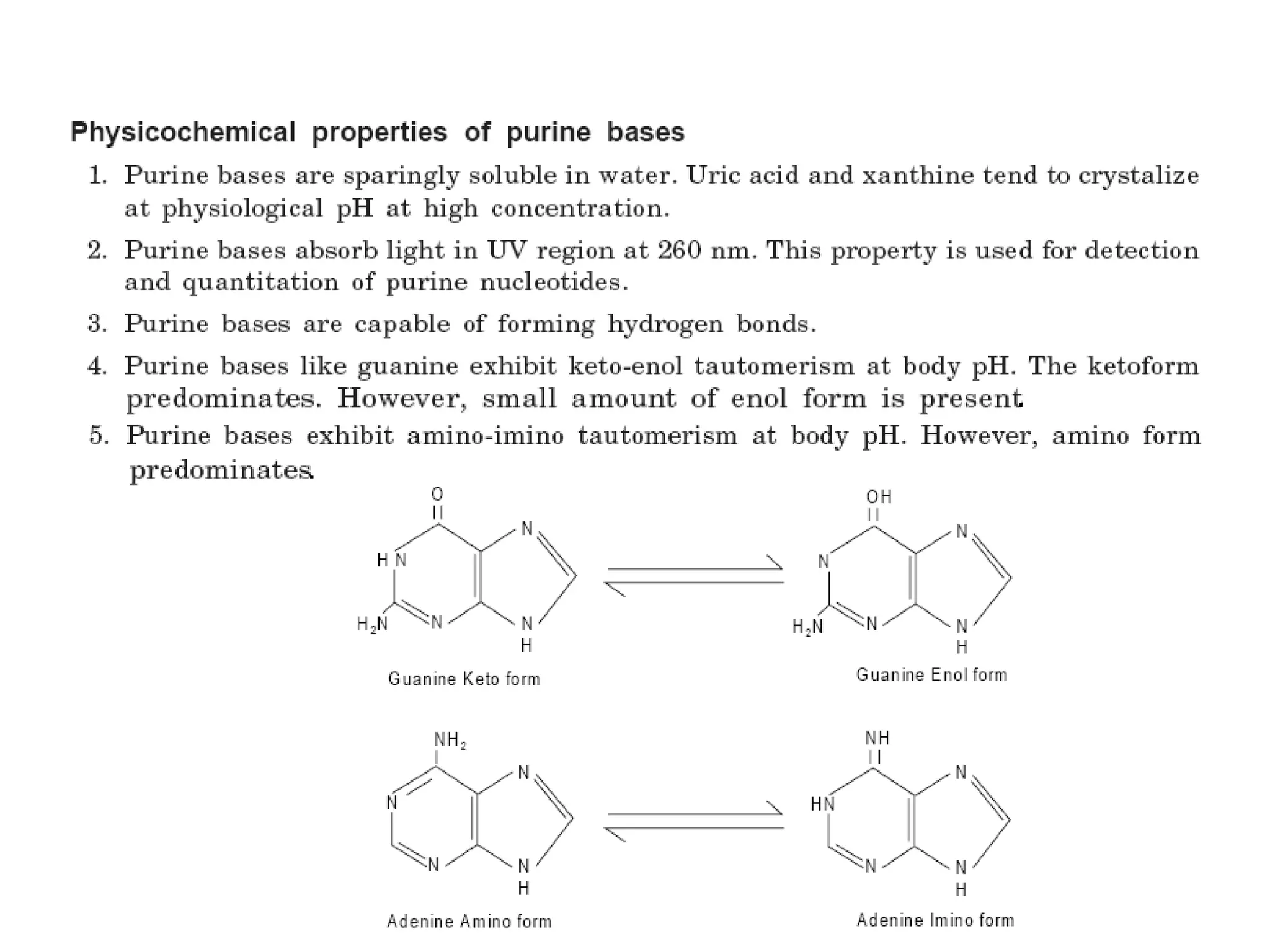
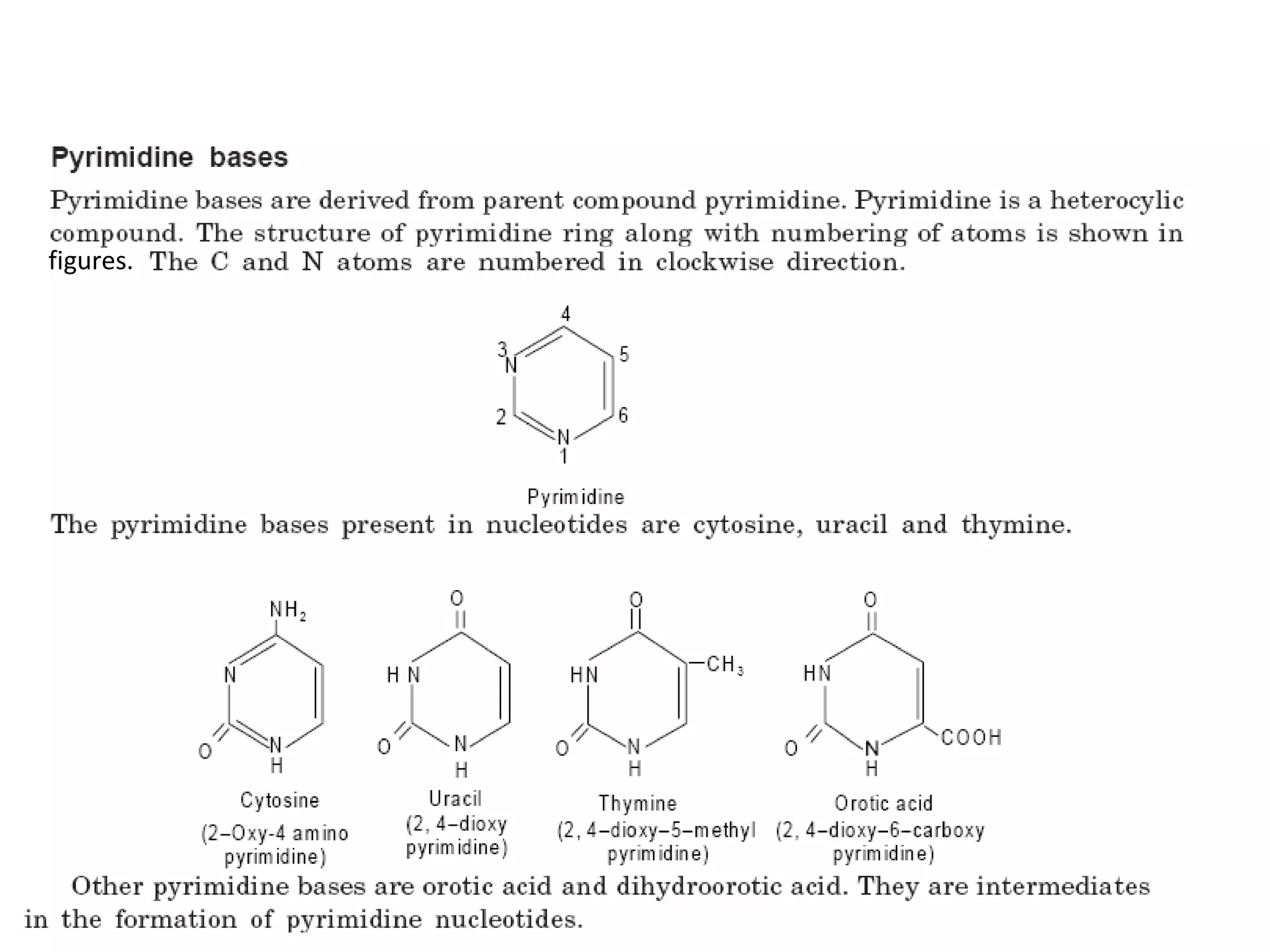
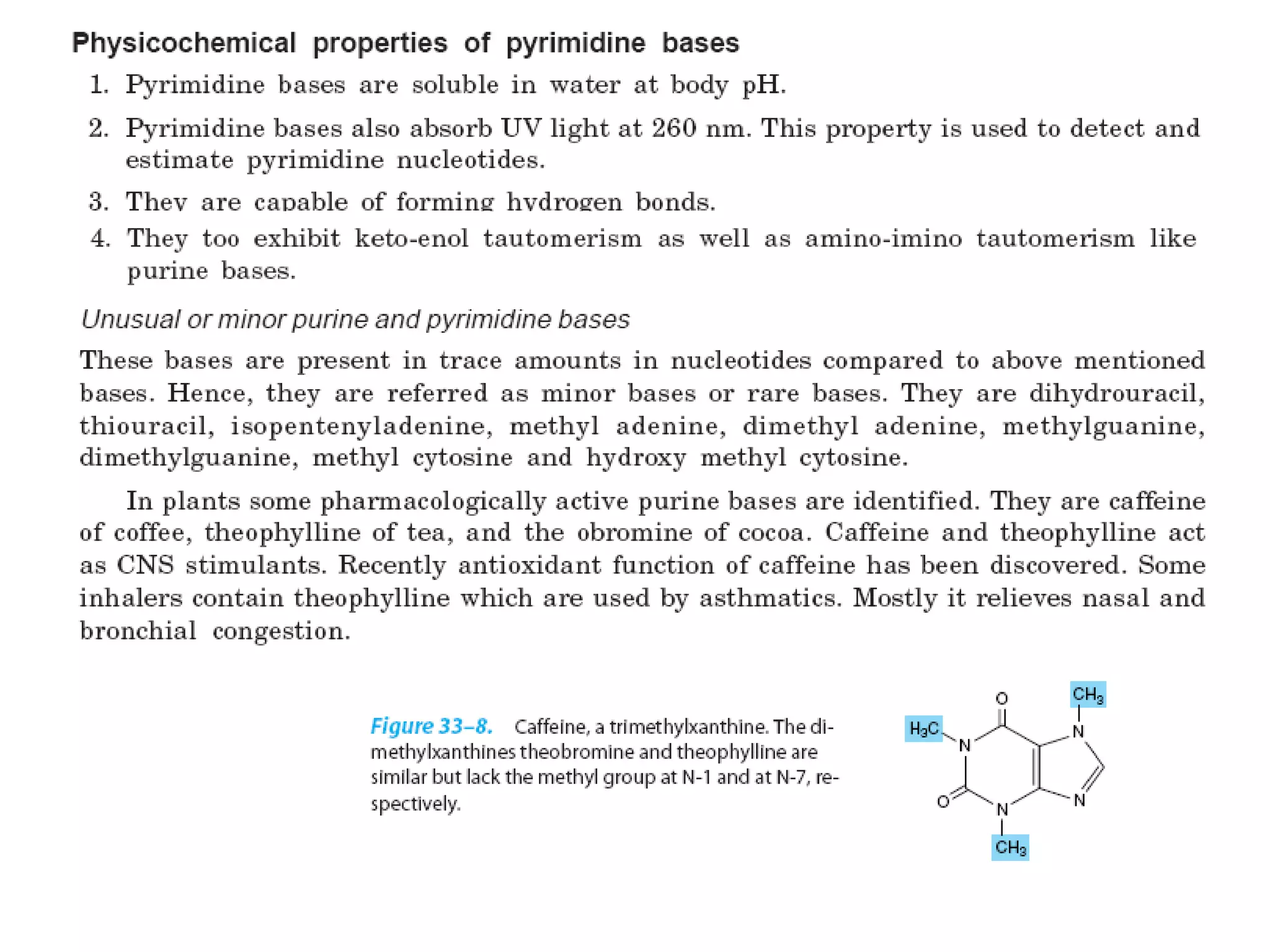


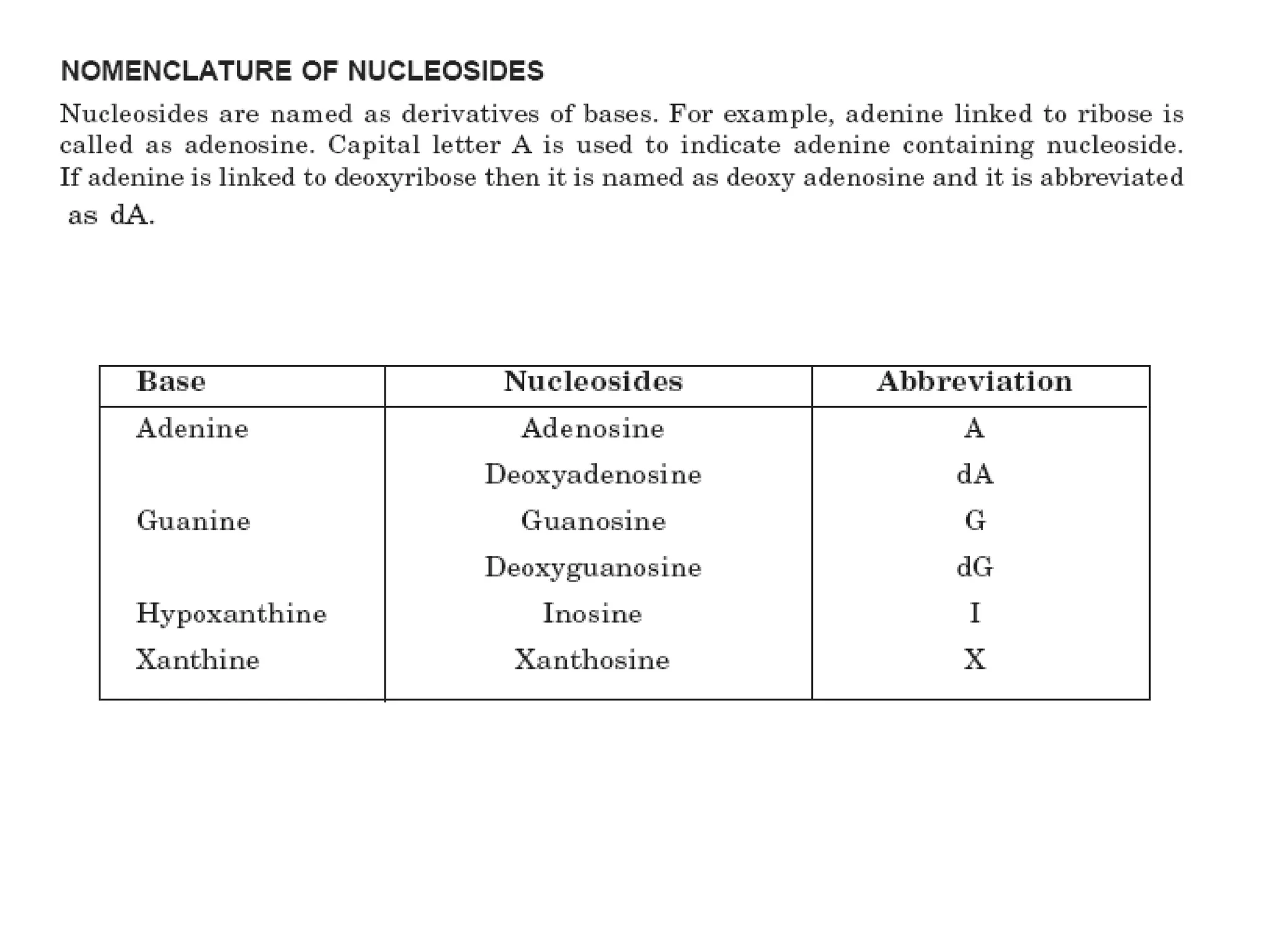
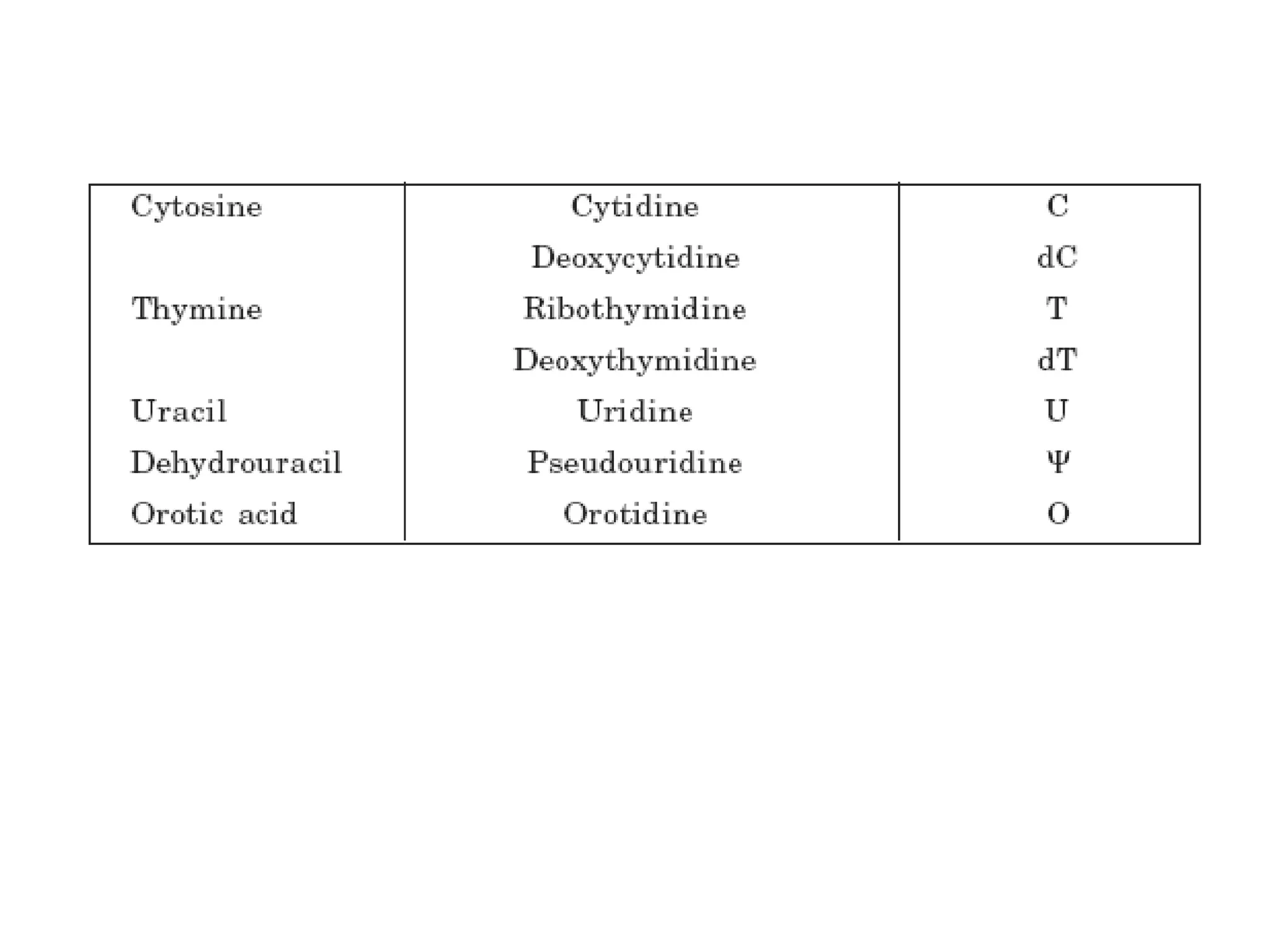
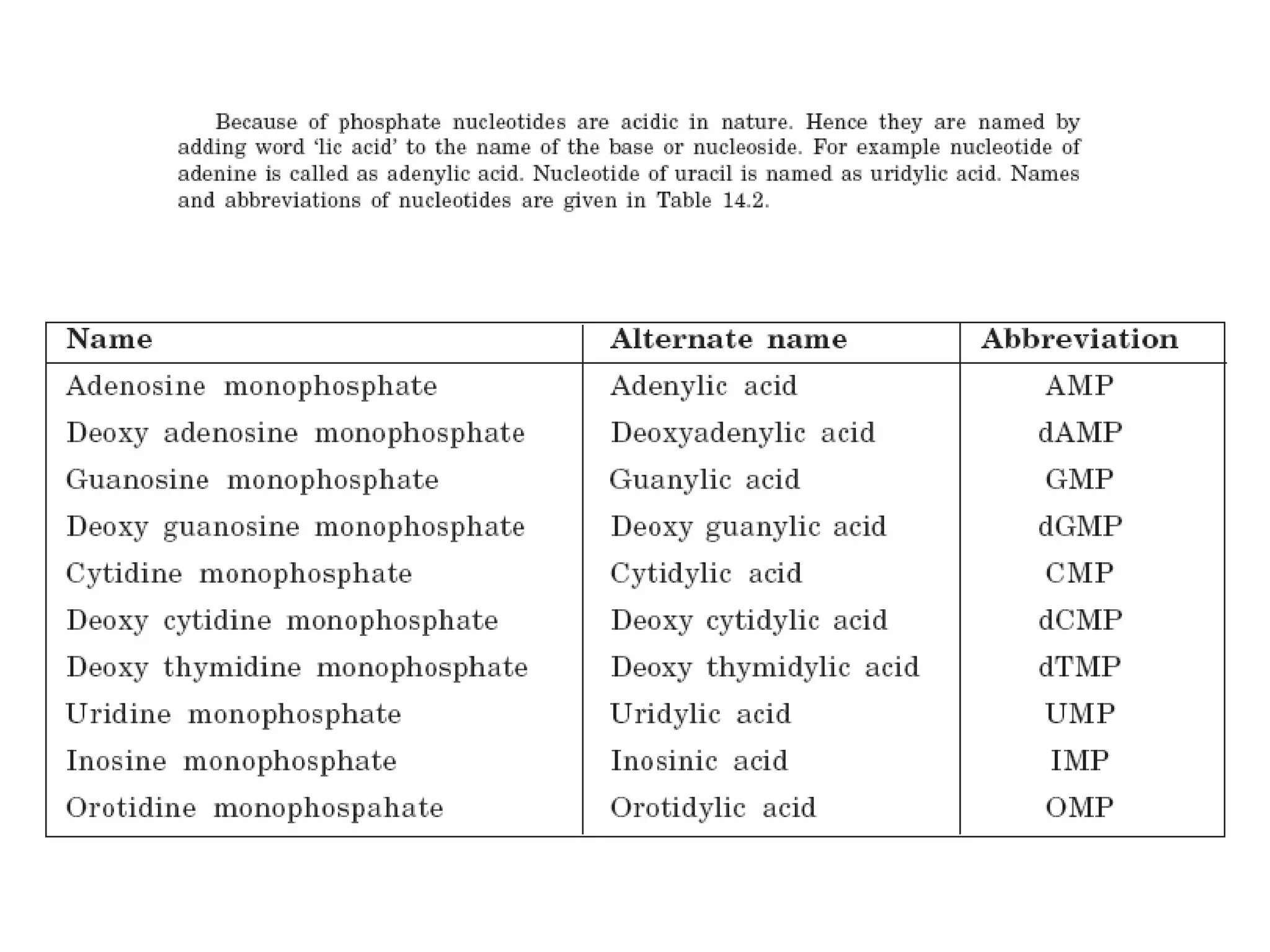
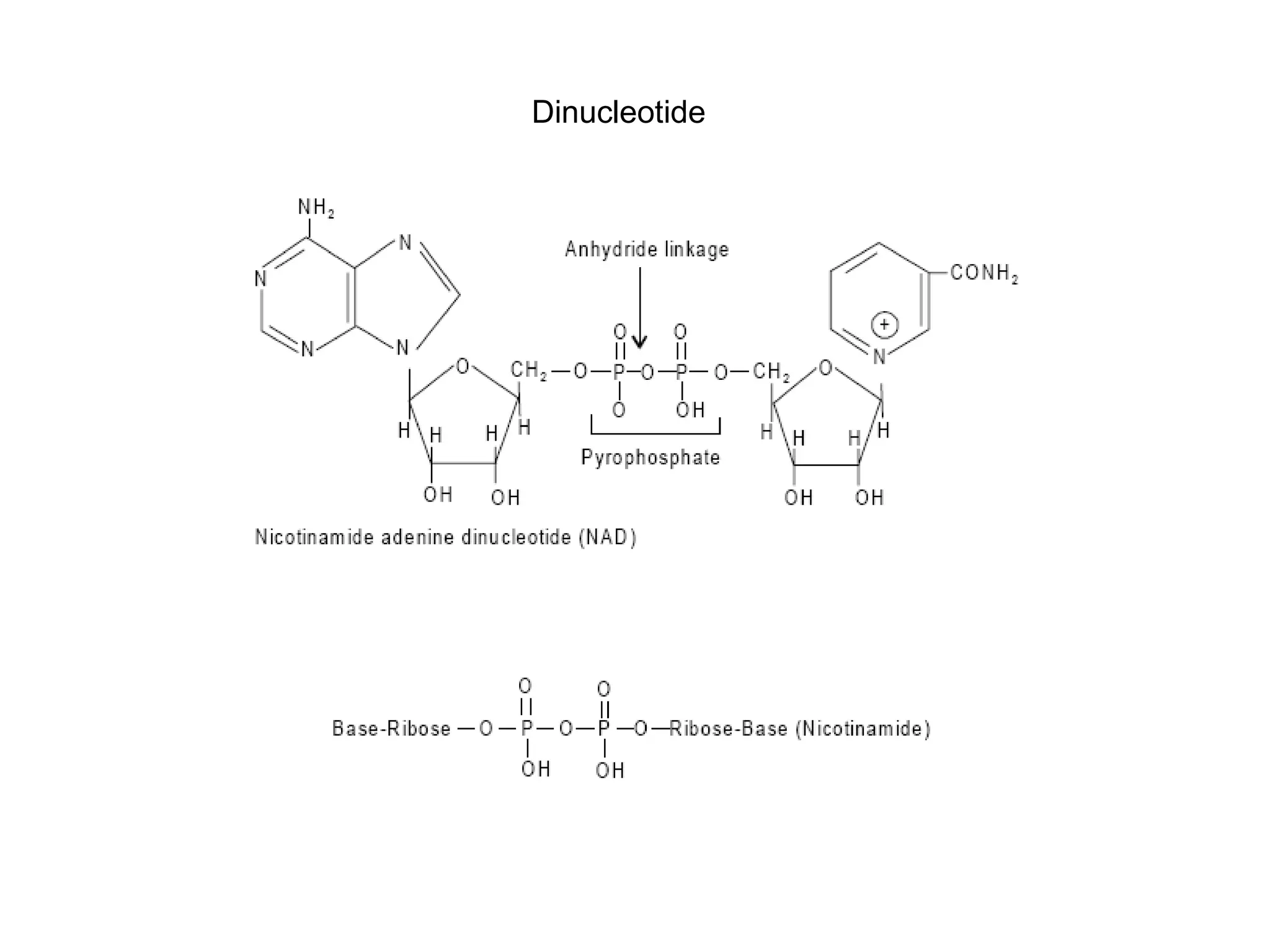
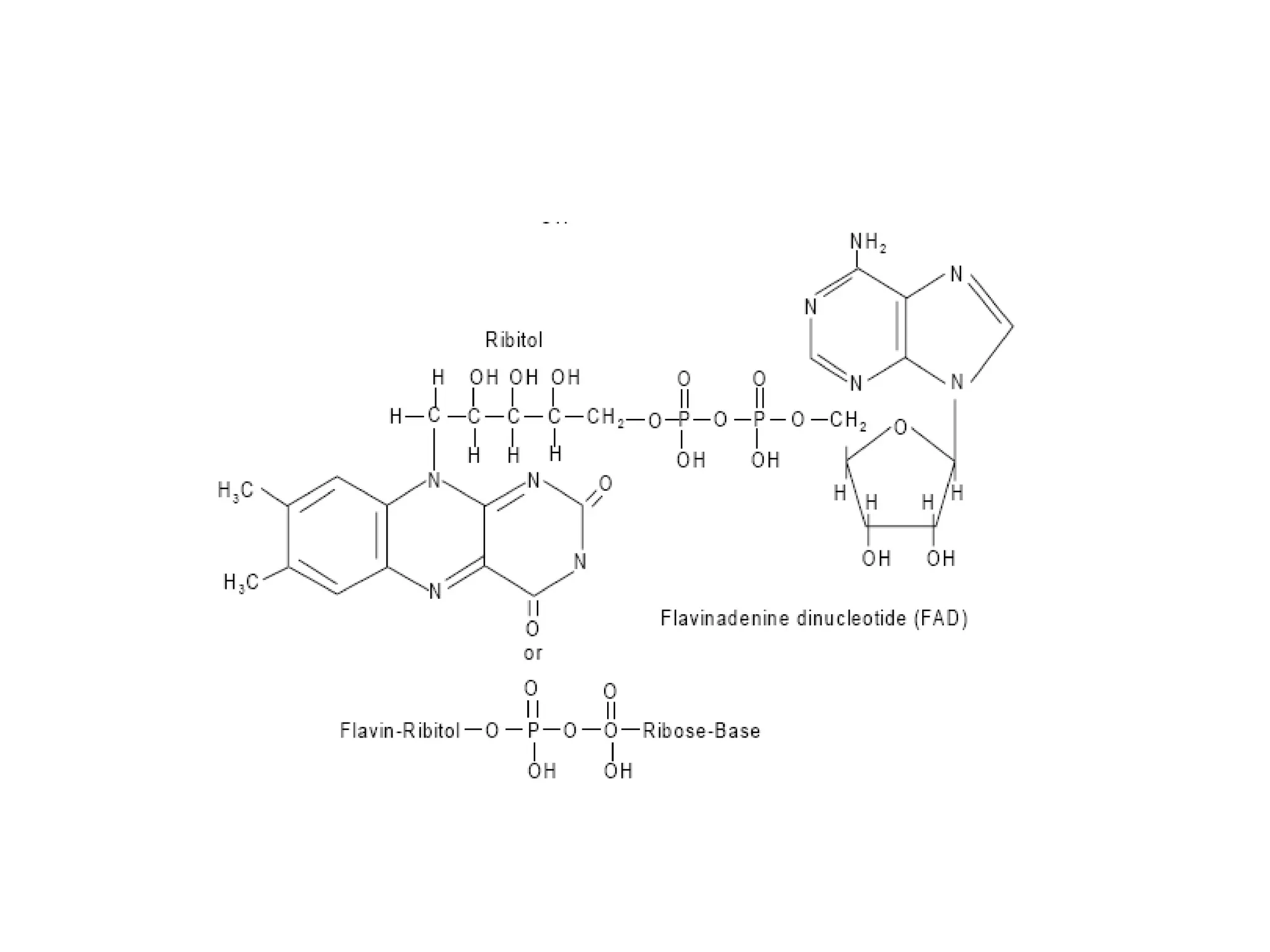


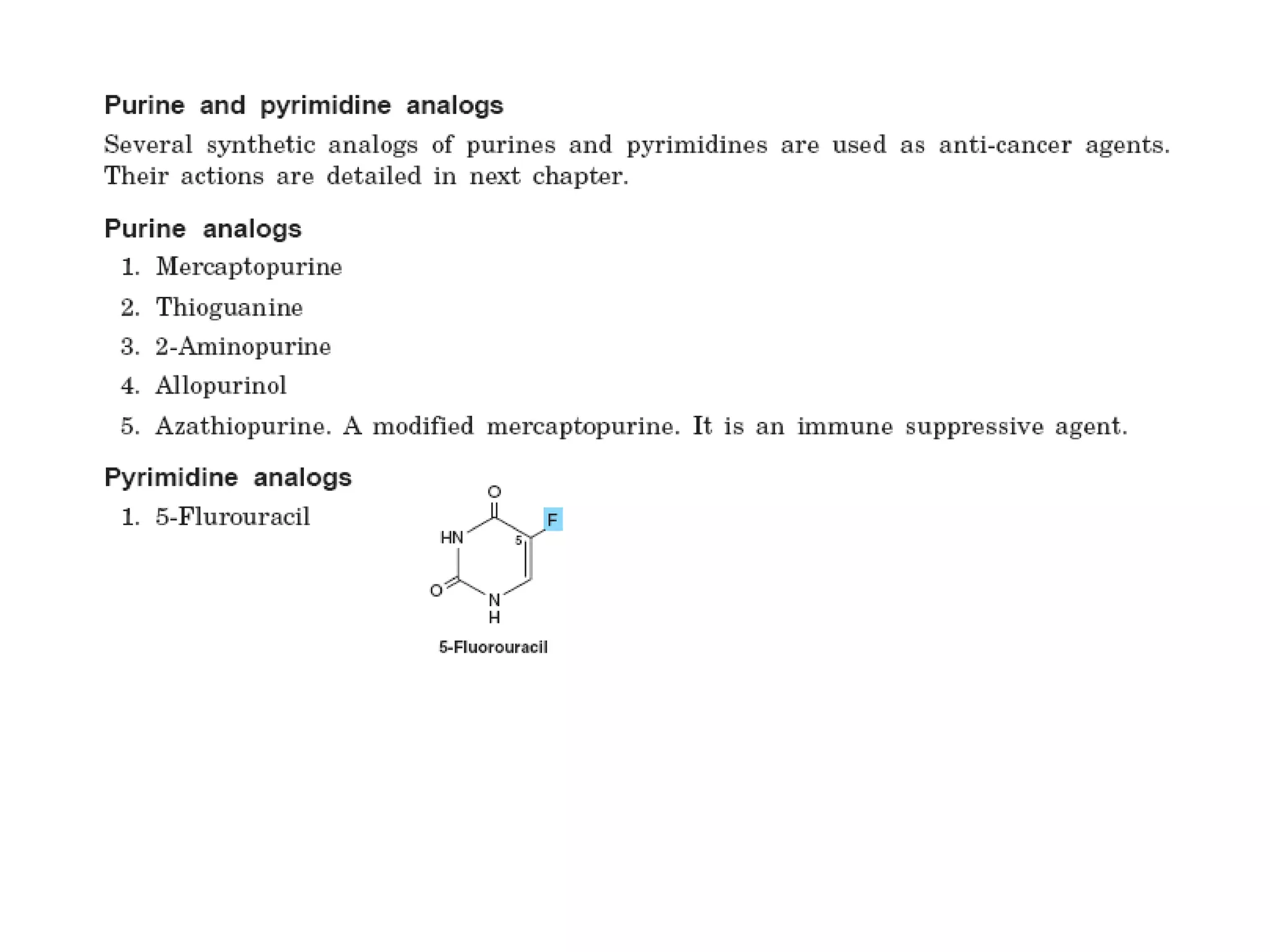
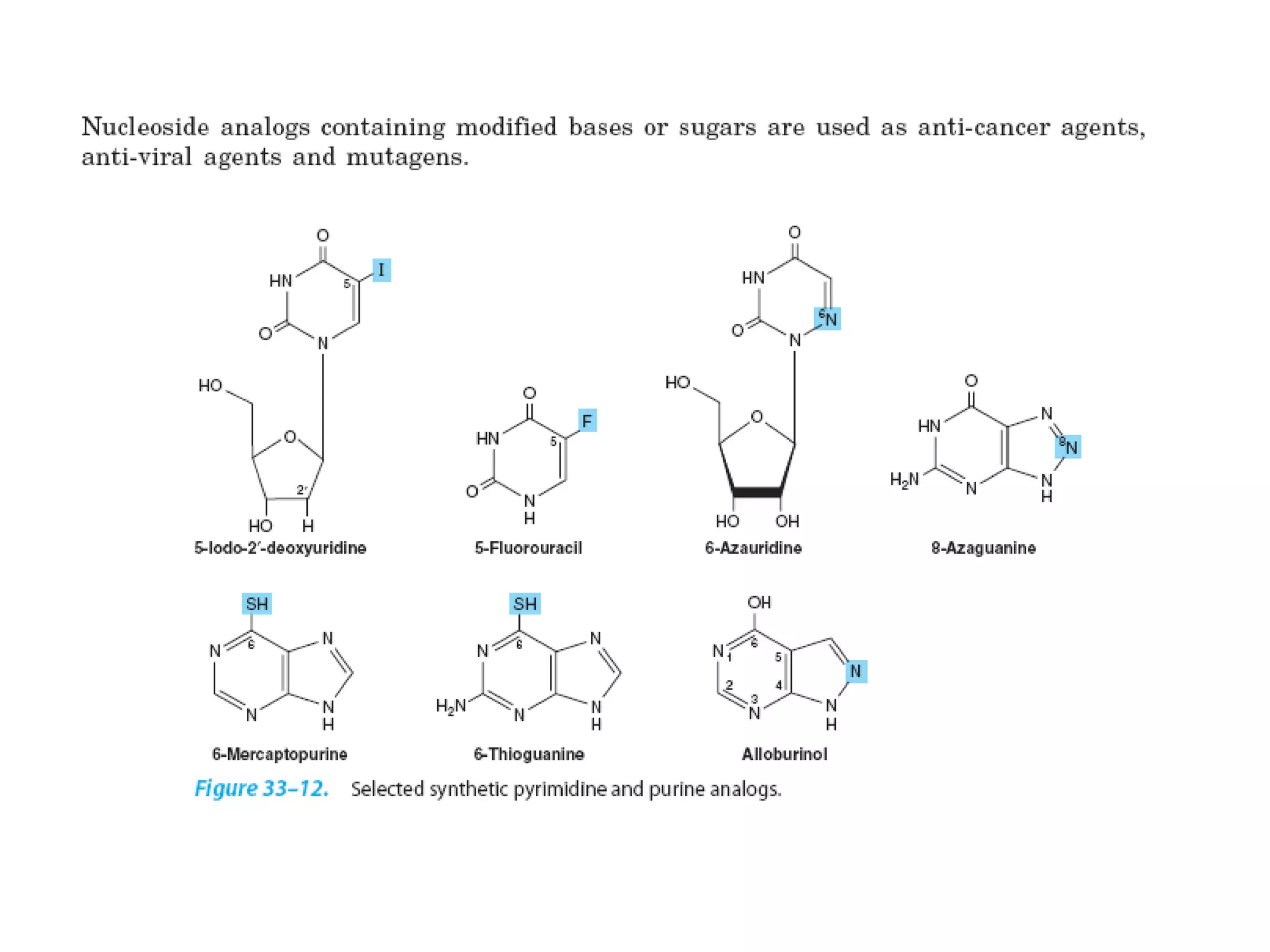


Nucleic acids are made up of monomeric units called nucleotides. Nucleotides serve important biochemical functions as components of coenzymes involved in transferring phosphoryl, sugar, lipid groups. They also function as second messengers like cAMP and cGMP. Synthetic nucleotides containing halogens or additional nitrogen are used in chemotherapy and immunosuppression. Purines and pyrimidines are heterocyclic nitrogen-containing compounds that form the bases of nucleic acids. Nucleosides are derivatives of bases linked to a sugar, while nucleotides contain a phosphoryl group attached to the sugar. RNA and DNA are made up of ribonucleotides and deoxyribonucleotides formed through a series of phosphorylation and conversion reactions in






























