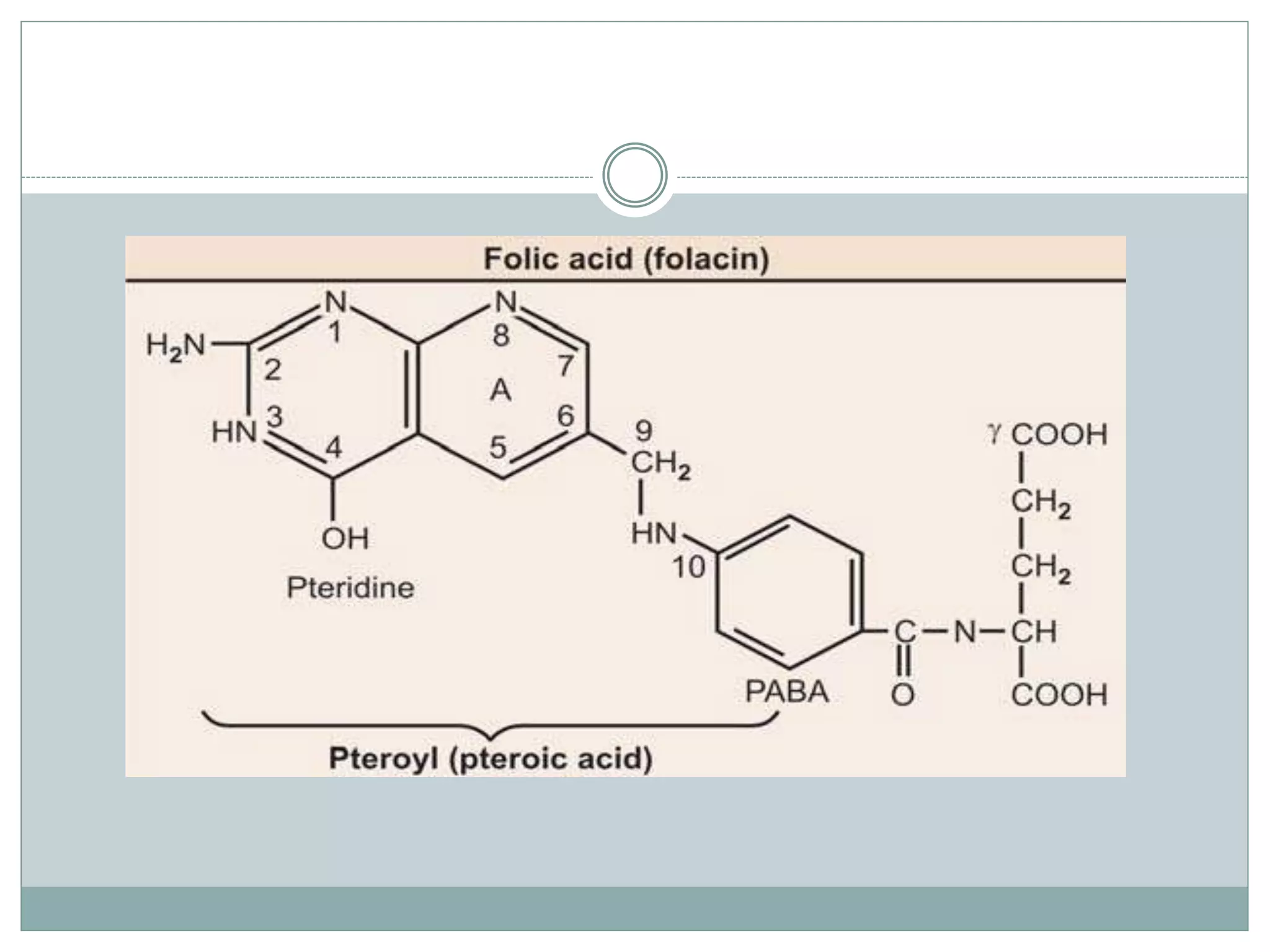
Folic acid, essential in one-carbon metabolism, plays a key role in biosynthesis and acts as a carrier of one-carbon groups. It exists in various forms and is converted into its active coenzyme form, tetrahydrofolate, through enzymatic reactions, supporting several metabolic pathways including amino acid metabolism and DNA synthesis. Deficiency can lead to megaloblastic anemia and other health issues, and is influenced by certain medications.