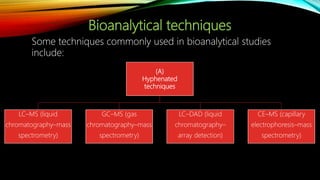
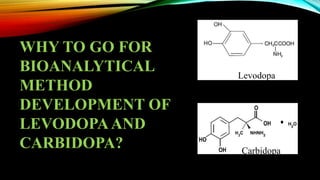
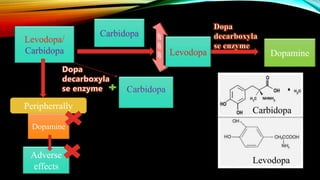
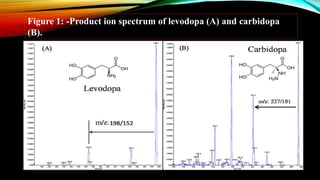
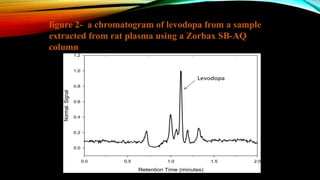
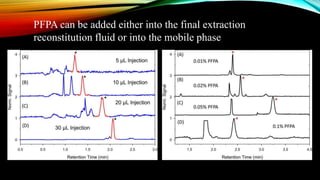
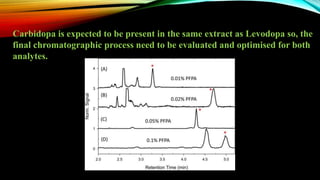
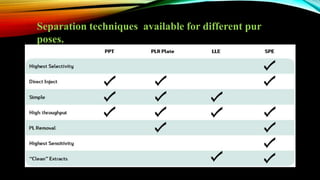
1) A bioanalytical method was developed and validated for the quantification of levodopa and carbidopa in rat plasma using LC-MS/MS. Derivatization and ion-pairing chromatography were used to improve the chromatographic retention of the polar analytes.
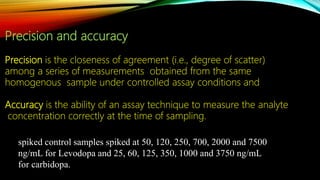
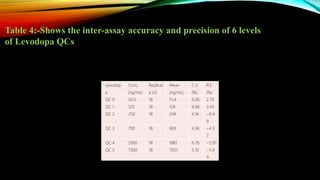
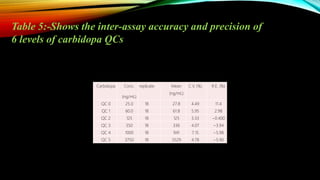
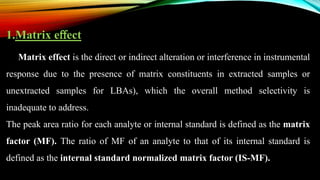
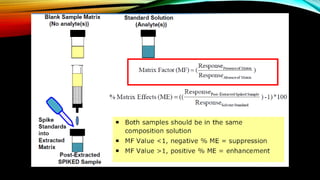
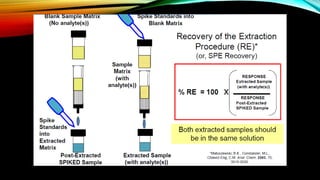
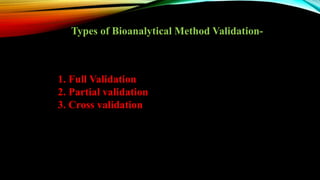
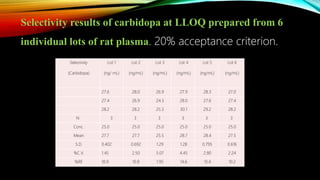
2) The method was fully validated as per FDA guidelines and demonstrated selectivity, linearity, accuracy, precision, recovery, matrix effects and stability in accordance with acceptance criteria.
3) The validated method was successfully applied to support toxicokinetic studies of levodopa and carbidopa in rats.





















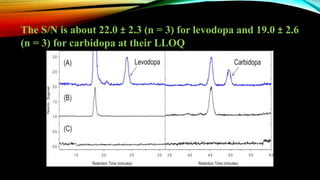
![2. Sensitivity
Sensitivity Is the lowest analyte concentration that can be
measured above the noise with acceptable accuracy and
precision (i.e., lower limit of quantification [LLOQ]).](https://image.slidesharecdn.com/presentation1-190401170444/85/Bioanalytical-method-development-and-validation-35-320.jpg)