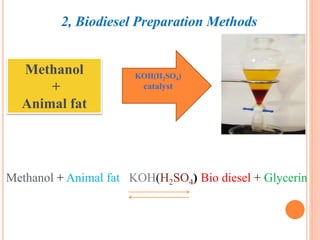
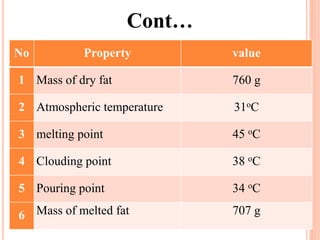
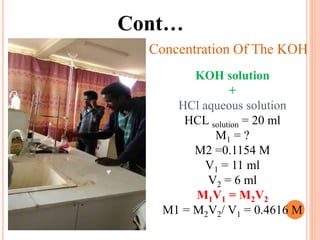
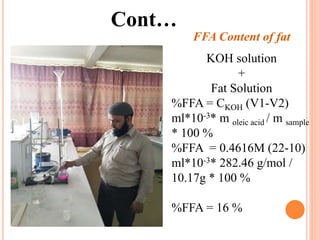
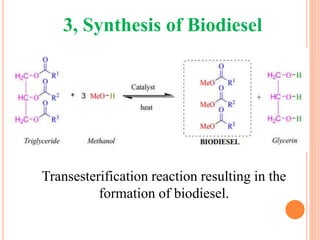
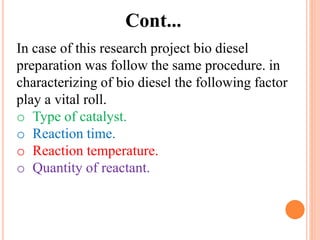
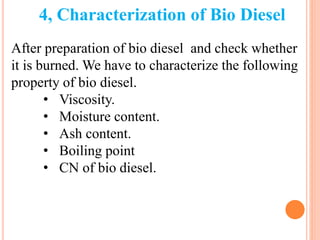
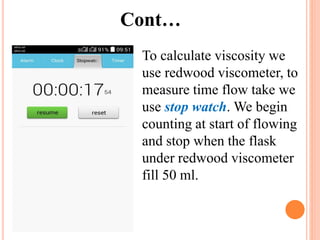
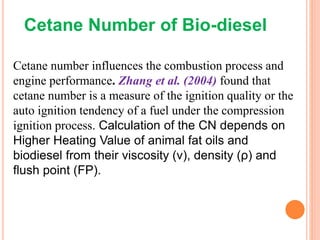
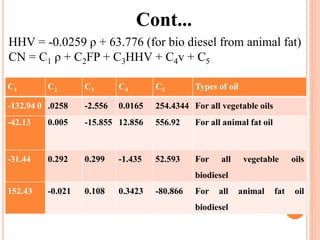
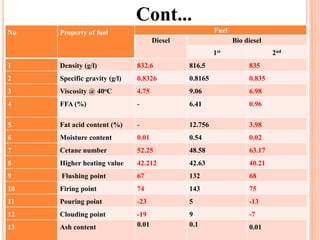
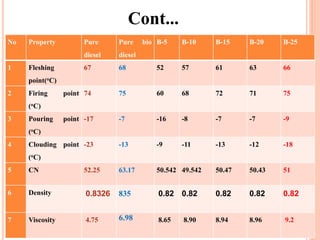
The document discusses the preparation of biodiesel from animal fat through transesterification. Specific objectives include processing and characterizing the animal fat and biodiesel. Biodiesel is prepared using a base catalyst method with KOH and methanol or an acid catalyst method with H2SO4 and methanol. The biodiesel is then characterized by testing properties such as viscosity, moisture content, ash content, boiling point, and cetane number. Comparing biodiesel properties to pure diesel and testing blends is also discussed. The methodology outlines animal fat processing, biodiesel preparation methods, and characterizing both the animal fat and resulting biodiesel.



![Introduction
Bio diesel was a renewable source of energy
Which substitute to fossil diesel fuel made from
biodegradable sources such as animal fat.
No or small engine modification are to made in
order to use biodiesel as a substitute of
conventional diesel.
In addition, biodiesel can be mixed with diesel in
many proportions. (Stephen Joseph) (2004)
[5]](https://image.slidesharecdn.com/biodiesel-191022101604/85/Bio-diesel-4-320.jpg)



![Literature Review
[1] Pizarro Lopes et.al (2011) Biodiesel is an alternative
fuel to fossil diesel that contributes to diversify energetic
sources, as well as to reduce greenhouse gas emissions.
[2] Fevzi Yasar et.al (2010) The viscosity and density of
biodiesel fuels are important parameters due to being key
fuel properties for injection and combustion process of
diesel engines.
[3] Beaton et.al (2012) transesterification of triglycerides
by methanol, ethanol, propanol, and butanol has proved
that it is currently the method of choice.](https://image.slidesharecdn.com/biodiesel-191022101604/85/Bio-diesel-10-320.jpg)