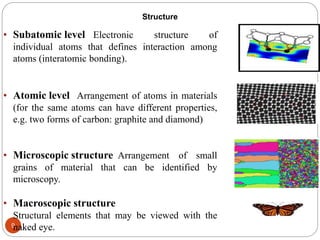
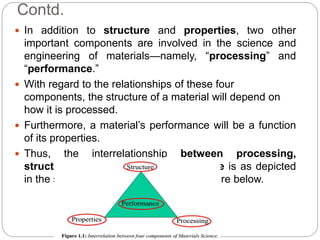
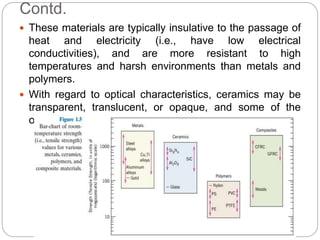
The document discusses the history of materials used by human civilization and the development of materials science and engineering. It describes how early civilizations progressed from the Stone Age to the Bronze Age to the Iron Age based on their ability to produce and use increasingly advanced materials. More recently, the development of advanced materials like ceramics, polymers, composites, and semiconductors has driven technological progress. The core components of materials science are described as structure, properties, processing, and performance, and how they interrelate.