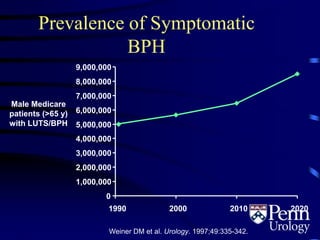
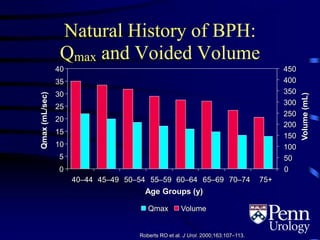
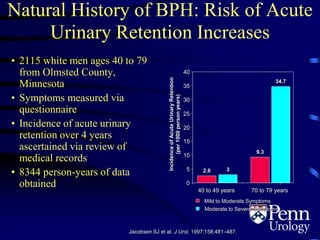
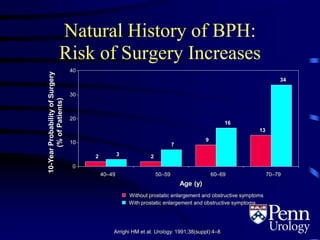
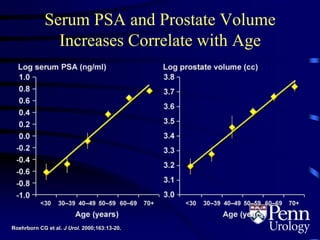
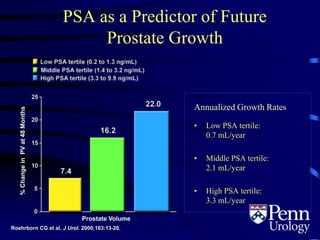
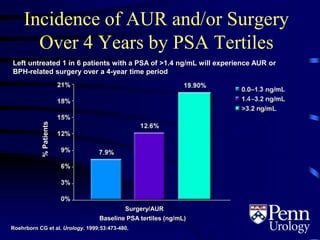
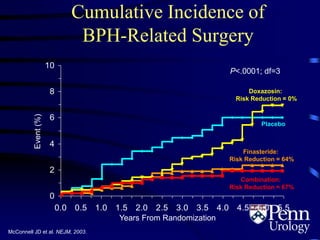
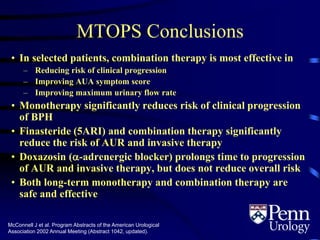
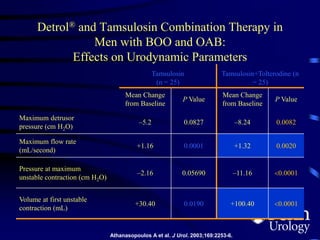
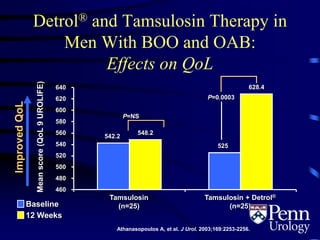
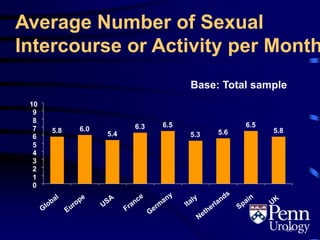
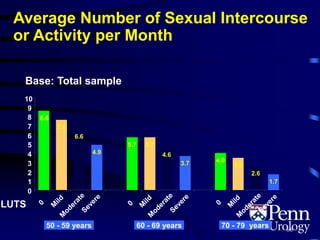
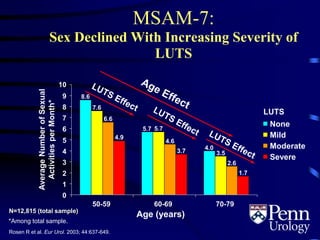
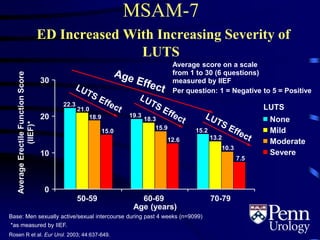
This document provides an overview of benign prostatic hyperplasia (BPH), including its epidemiology, terminology, evaluation, medical and surgical treatment options, and pathophysiology. Some key points include:
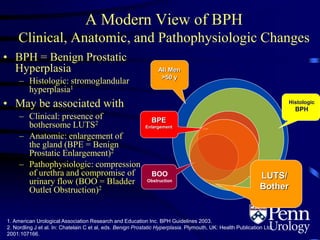
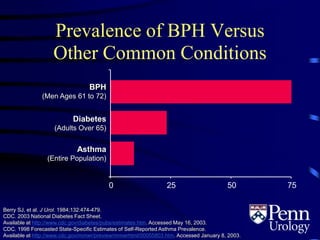
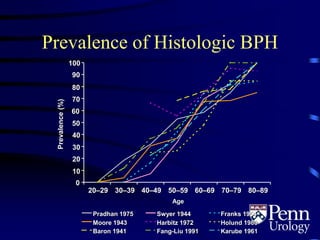
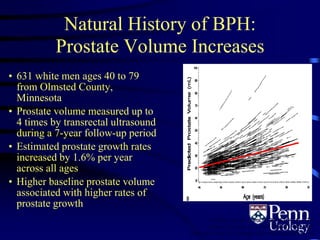
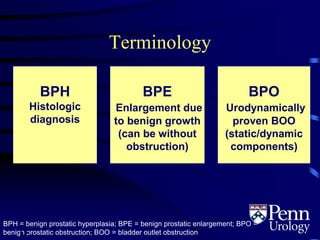
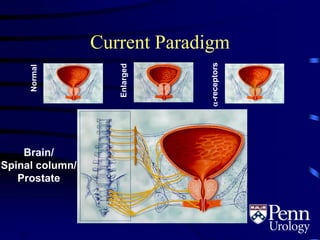
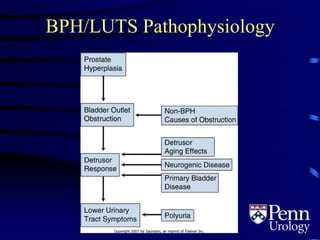
- BPH is a histological diagnosis defined as nonmalignant hyperplasia of the prostate gland. It often presents clinically as bothersome lower urinary tract symptoms and an enlarged prostate.
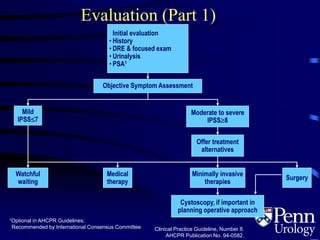
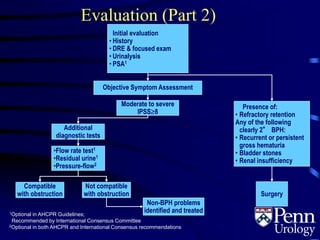
- Evaluation involves medical history, physical exam including DRE, urinalysis, and symptom assessment. Additional tests like flow rate and residual urine may be used if symptoms are moderate to severe.
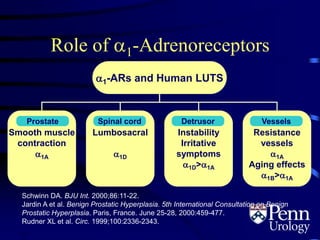
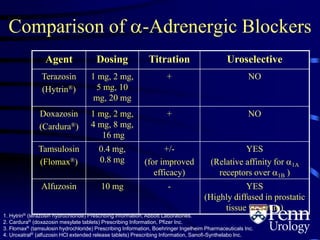
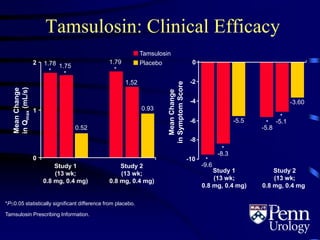
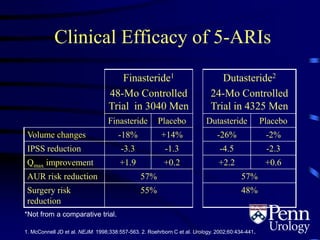
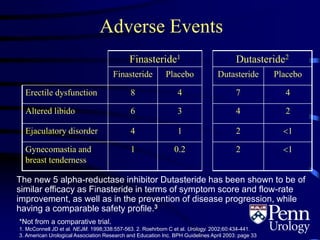
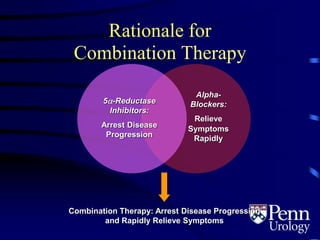
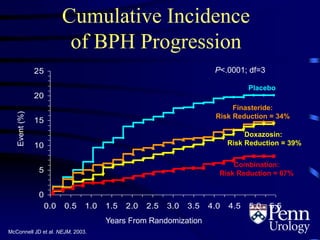
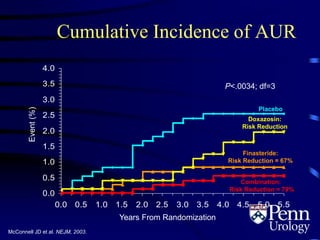
- Medical therapy options are alpha-blockers which target the dynamic component of obstruction, and 5-alpha-re